Knowledge Gaps
 Much has been accomplished in understanding the interplay of MAP and its host in recent years using as new tools the genome of MAP and the many cellular, proteomic and reagents and assays recently developed. Most MAP research is done on cattle and studies specifically on goats are very few. Thus, sometime we have to make assumptions about how MAP infection progresses in goats. These are some of the core questions about MAP pathology and pathogenesis that remain unanswered:
Much has been accomplished in understanding the interplay of MAP and its host in recent years using as new tools the genome of MAP and the many cellular, proteomic and reagents and assays recently developed. Most MAP research is done on cattle and studies specifically on goats are very few. Thus, sometime we have to make assumptions about how MAP infection progresses in goats. These are some of the core questions about MAP pathology and pathogenesis that remain unanswered:
- What is the minimally infective dose by animal species and age?
- Why are young ruminants much more susceptible than adult ruminants?
- What proportion of infected animals recover (clear the infection), if any?
- What factors affect MAP virulence?
- Are all MAP strains equally virulent?
- What are the innate immunologic responses to MAP infection in young animals?
- Continued progress in comprehending host-MAP and MAP-environment dynamics is being made. This page summarizes our current knowledge about the impact of a MAP infection on goats.
Infection

The target for MAP is the goat’s gastrointestinal (GI) tract. The end of the small intestine, called the ileum, is the primary site for infection. Goats swallow the organism (via MAP-contaminated milk, water or feed) which then invades through the intestinal wall localizing in specialized tissues called Peyer’s patches where it is taken up by the goat’s immune cells (macrophages). This microscopic infection of macrophages in the small intestinal persists for years without triggering any systemic response from the animal’s immune system, meaning that the animal is MAP-infected but isn’t sick and isn’t responding to the infection in any detectable way. At some point MAP spreads beyond the intestinal tract to lymph nodes flanking the GI tract (the mesenteric nodes). Later, for reasons not yet understood, the infection spreads throughout the goat at which point it can infect the unborn fetus. Clinical symptoms of Johne’s disease usually begin to appear at this point. When goats become thin due to the damage caused to their intestinal tract by MAP and the resulting inflammation, diagnostic tests are almost invariably positive, whether testing by fecal culture, fecal PCR, or by ELISA for serum antibodies.
Throughout this long sub-clinical phase (roughly 2-10 years) when the goat appears healthy although MAP-infected, it is capable of transmitting the infection by shedding MAP in milk and manure. Initially, the MAP bacteria may be only intermittently detectable but as the infection progresses, animals become steady shedders of MAP in feces.
Inflammation
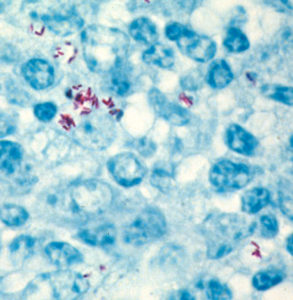
MAP employs a variety of strategies to make sure it gets to where it can persist, and more importantly replicate, in an animal. One of these strategies is to use a component of the animal’s defense system for its own home. After having been swallowed and travelled down the small intestine, MAP is picked up by host cells (with the intention of killing the foreign invader) and is carried inside these cells into the wall of the GI tract landing in the Peyer’s patches (lymphoid tissue similar to tonsils) and sets up residence within macrophages (white blood cells) localized in that region and design to fight infection. Through complex mechanisms, MAP somehow turns off the bacteria-fighting mechanisms of the macrophages and instead creates a hospitable environment for itself. Far from alarming the immune system, this invasion seems to be ignored by the goat’s immune system or actively suppressed by MAP. No detectable pathology appears at this phase. The photo on the right shows the red-stained MAP bacteria surrounded by a limited number of blue-colored goat inflammatory cells.
At some point, the goat mounts a stronger inflammatory response. Inflammation is defined as a protective tissue response to injury or destruction of tissues, which serves to destroy, dilute, or wall off both the injurious agent and the injured tissues. Through production of various cytokines (gamma interferon being an important one) the body begins this so called “cell-mediated” immune response. More macrophages are recruited to the site of infection and the result is called granulomatous inflammation, an aggregate of living, dying and dead MAP plus white blood cells from the goat, namely macrophages and lymphocytes. This lesion progresses, but remains a localized battle in the gastrointestinal tract – – – for a while.
 As more MAP enter and replicate in the macrophages however, and as more cells are recruited to fight them, the lesion expands. This granulomatous inflammation spreads, and the infected macrophage may then leave the gastrointestinal tract inside macrophages and be filtered out by the neighboring lymph nodes. Eventually, the goat loses it’s battle with MAP and the bacteria spread through the blood to other organ systems.
As more MAP enter and replicate in the macrophages however, and as more cells are recruited to fight them, the lesion expands. This granulomatous inflammation spreads, and the infected macrophage may then leave the gastrointestinal tract inside macrophages and be filtered out by the neighboring lymph nodes. Eventually, the goat loses it’s battle with MAP and the bacteria spread through the blood to other organ systems.
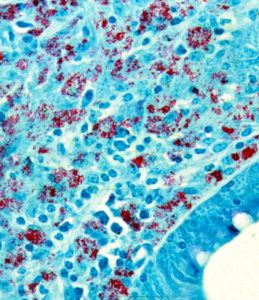
In many respects the lesion resembles that of leprosy (i.e., lesions produced by infection with Mycobacterium leprae) more than that of tuberculosis (caused by Mycobacterium tuberculosis). As in leprosy, some lesions may have numerous acid-fast (red) bacteria. Such lesions as shown on the left are referred to as lepromatous or multi-bacillary. When very few MAP bacteria are seen it is called paucibacillary.
 This uncontrolled spreading inflammation is the primary reason Johne’s disease is fatal. The GI tract is severely damaged and is no longer capable of absorbing nutrition. So, the goat keeps eating well but steadily loses weight. MAP-infected goats sometimes have pasty feces, but it is not like the
This uncontrolled spreading inflammation is the primary reason Johne’s disease is fatal. The GI tract is severely damaged and is no longer capable of absorbing nutrition. So, the goat keeps eating well but steadily loses weight. MAP-infected goats sometimes have pasty feces, but it is not like the
diarrhea seen in cattle.
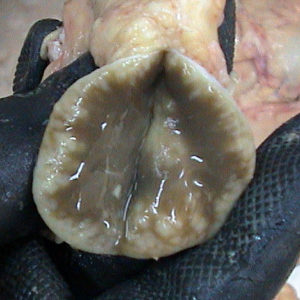
In this latter stage of infection the intestine is somewhat thickened. More noticeable, however, are the enlarged lymph nodes adjacent to the GI tract. When cut in half, these swollen lymph nodes often reveal pale whitish areas that are collections of white blood cells (macrophages and T-lymphocytes) that have come to the MAP infection site to try and deal with the invading microbe. The lymph node from a Saanen goat in Chile diagnosed with Johne’s disease is shown on the right. Normally the lymph node with be a uniform, liver-like color. The white areas are all sites of granulomatous inflammation. Sometimes these lesions will be calcified.
For specialists
One of the best publications on paratuberculosis pathology in goats is by J.M. Corpa et al. Classification of lesions observed in natural cases of paratuberculosis in goats. Journal of Comparative Pathology 122:255-265, 2000. Those authors clearly show that there is a range of pathology types. They found that 50% of 68 goats with paratuberculosis had diffuse multibacillary lesions with acid-fast bacteria (AFB) seen in both Peyer’s Patches (gut lymphoid tissue) and in the mucosa (superficial layer of the gut): what most would consider the classical or typical paratuberculosis pathology. However, 24% (16/68) had focal lesions with AFB seen Peyer’s Patches of 1 and in mucosa for 6 of these 16 goats. Another 15% (10/68) had diffuse lymphocytic inflammation and AFB were few or absent, i.e. paucibacillary lesions. Lastly, 8 goats had diffuse mixed lesions and AFB were few in number but always present.
This important publication highlights the diversity of pathological responses and illustrates that MAP bacteria cannot always be seen in the lesions, an observation relevant to the controversy about absence of visible MAP in pathology of Crohn’s disease.

