The story of Johne’s disease for non-experts
Michael T. Collins, DVM, PhD, DACVM
Where it all started and why the funny name?

“Hey Doc! While you’re here, would you please look at Bella? She had a nice calf about three weeks ago and has been eating well, but she looks too thin and isn’t milking as well as she should. She also seems to have rather loose manure.”
This is the kind of question I imagine was presented to Dr. F. Harmes about a Guernsey cow in the Oldenburg region of Germany in 1895. Dr. Harmes preliminary diagnosis was intestinal tuberculosis (TB). TB in cattle was quite common in Germany then. But when he did the tuberculin skin test to confirm his diagnosis, the cow tested negative. So, the reason for the cow’s condition remained a mystery.

A few months later, the cow died. Curious as to what killed the cow, Dr. Harmes sent intestines and other tissues to the Pathology Unit at the veterinary school in Dresden. There the tissues were examined by Dr. Heinrich A. Johne, Professor of Pathology (picture at right provided by Dr. J.B. Jørgensen, State Veterinary Serum Laboratory, Copenhagen, Denmark), and Dr. Langdon Frothingham, a visiting scientist from the Pathology Unit in Boston, Massachusetts.
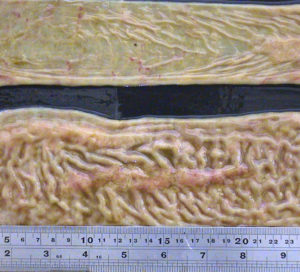 They observed that the small intestine was quite a bit thicker than expected and that lymph nodes near this thick intestine were enlarged. The photo on the left shows a normal intestine at the top and the intestine thickened due to Johne’s disease at the bottom. Lymphoid tissue, called Peyer’s Patches, are also quite prominent (the raised and slightly red tissue running long-ways down the center of the thickened intestine). Interestingly, Dalziel in 1913 saw the same kind of pathology when he removed a section of intestine from a person with Crohn’s disease remarking in his report that it resembled the cattle problem Dr. Johne had described.
They observed that the small intestine was quite a bit thicker than expected and that lymph nodes near this thick intestine were enlarged. The photo on the left shows a normal intestine at the top and the intestine thickened due to Johne’s disease at the bottom. Lymphoid tissue, called Peyer’s Patches, are also quite prominent (the raised and slightly red tissue running long-ways down the center of the thickened intestine). Interestingly, Dalziel in 1913 saw the same kind of pathology when he removed a section of intestine from a person with Crohn’s disease remarking in his report that it resembled the cattle problem Dr. Johne had described.
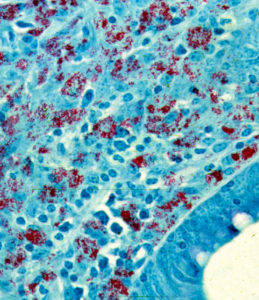
Using what at the time were newly developed histopathology techniques, parts of the intestine were “fixed” (pickled in formaldehyde), sliced into very thin sections, placed on a microscope slide, and stained with special dyes – known as an acid-fast stain – designed to help visualize bacteria of the type causing TB. Under the microscope, Drs. Johne and Frothingham saw that the intestinal wall was filled with inflammatory cells of the kind to be expected in TB (macrophages and lymphocytes – the blue-colored stuff in the photo). In addition, they saw abundant red-staining bacteria (which microbiologists call acid-fast bacteria) throughout the inflamed tissues. Basically, it looked just like intestinal TB. But, when a sample of the fresh infected tissue containing the red-staining bacteria was injected into guinea pigs, it didn’t cause TB. This took place shortly after Louis Pasteur had devised the “germ theory” of disease and before techniques for growing bacteria in the laboratory were widely available. Inoculating animals, therefore, was a routine way of detecting infectious microbes such as those that cause TB, and guinea pigs are quite susceptible to tuberculosis. So, the diagnosis on this cow remained a mystery.
Drs. Johne and Frothingham concluded that the disease seen in the very sick Guernsey cow was caused by a bacterium other than the one normally causing TB in cattle, namely Mycobacterium bovis. They speculated that perhaps the pathology was due to a related bacterial pathogen such as the one causing TB in birds, aptly named Mycobacterium avium. Considering their subject’s gross pathology, microscopic pathology (histopathology) and animal inoculation findings, they proposed the name “pseudotuberculous enteritis” for the disease; a designation meaning inflammation of the intestine resembling intestinal TB but not actually the same as intestinal TB – somehow different. Soon after publication of their report, veterinarians began reporting outbreaks of this curious intestinal malady among dairy cows in Denmark, The Netherlands and elsewhere in continental Europe.
Hopping across the Atlantic
Dairy cattle came to the U.S. with the first European settlers. However, the dairy industry really started growing in the U.S. with the invention of the mechanical milking machine. As the dairy industry expanded, dairy cattle of diverse breeds and with high milk production potential were imported to the U.S. to build dairy herds using animals with the best genes. Most of these came from Europe, home to the major dairy breeds of cattle such as Holsteins, Guernsey’s, Jerseys, Ayrshires, and Brown Swiss. Along with these imported cows, unknowingly and unfortunately, came a few that were incubating this mysterious malady that Dr. Johne had described.
 In the early 1900s, Pennsylvania was home to a good proportion of all U.S. dairy cows, and it was there that this strange phenomenon of diarrhea and weigh loss was first recognized. In 1908, replicating what had been observed earlier in Germany, a farmer noticed a cow that was very thin and suffering from diarrhea. Seeking a diagnosis, the farmer sent this cow to the state veterinary school for examination by pathologists. Dr. Leonard Pearson (pictured to the right), who was serving as Dean at the University of Pennsylvania Veterinary School, examined this cow and became the first to publish a U.S.-based report of what we now call Johne’s (Yo-nees) disease. Today the disease also goes by the name paratuberculosis.
In the early 1900s, Pennsylvania was home to a good proportion of all U.S. dairy cows, and it was there that this strange phenomenon of diarrhea and weigh loss was first recognized. In 1908, replicating what had been observed earlier in Germany, a farmer noticed a cow that was very thin and suffering from diarrhea. Seeking a diagnosis, the farmer sent this cow to the state veterinary school for examination by pathologists. Dr. Leonard Pearson (pictured to the right), who was serving as Dean at the University of Pennsylvania Veterinary School, examined this cow and became the first to publish a U.S.-based report of what we now call Johne’s (Yo-nees) disease. Today the disease also goes by the name paratuberculosis.
The clinical signs of paratuberculosis – diarrhea and weight loss – are a bit vague, resembling those of many other cattle diseases. The pathology, however, is quite distinctive, which makes diagnosis by necropsy and histopathology quite straight-forward, especially when special stains are used to reveal the acid-fast (red-staining) bacteria. What eluded diagnosticians for several years was the ability to grow the microbe causing this new disease in the laboratory. This, after all, has long been the most common and reliable way of confirming the diagnosis of an infection.
Growing MAP in the lab
In the golden days of microbiology, once Pasteur had shown that germs cause disease and Koch had devised a method to grow bacteria in pure culture, the causes of many of the worst diseases plaguing humans – such as tuberculosis, typhoid fever, cholera, and diphtheria – were identified. The scientific standard for proving that the microbe scientists recovered from patients was in fact the cause of a given disease was to 1) show that the organism was abundant in sick patients or animals but not found in healthy ones, 2) grow the organism in pure culture from diseased tissues, 3) reproduce the disease by inoculating a culture of the microbe it into healthy animals, thereby causing the same disease, and finally 4) recover the same bacterium from the sick or dead animals. This standard of scientific proof became known as Koch’s postulates after Robert Koch, the German microbiologist who formulated them. Koch soon abandoned these rigid criteria for proving causality after observing that some people were asymptomatic carriers of pathogens causing typhoid fever (remember Typhoid Mary?) and cholera.
For the next 17 years after Drs. Johne and Frothingham diagnosed their peculiar form of intestinal tuberculosis, the microbe causing this disease could be seen in tissue sections, but not cultured in the lab. Thus, it was not yet possible to figure out the identity of the microbe responsible for causing the thickened intestines, weight loss and diarrhea.
Finally, in 1912, a serendipitous observation by F. W. Twort, a British scientist, led to the successful isolation of the elusive microbe. Ironically, Twort’s success derived in part from his failure to do a careful job cleaning laboratory glassware, along with his discriminating eye. Thus, Dr. Twort observed the presence of small bacterial colonies growing like satellites around larger colonies in old bacterial cultures that he was preparing to discard. These larger colonies were contaminants in his cultures, a species of Mycobacterium common in the environment, called Mycobacterium phlei. Suspecting that the M. phlei bacteria were providing some essential nutrient, Twort incorporated a heat-killed preparation of M. phlei into his culture medium. This new culture medium, he discovered, supported the growth of a new acid-fast bacterium from intestinal tissues of cows with Johne’s disease. He named it “Mycobacterium enteriditis chronicae pseudotuberculosae bovis Johne” – a real mouthful. Fortunately for those biologists who pursued this microbe – as well as for anyone reading this article! – this name was soon shortened to Mycobacterium paratuberculosis, which, by microbiology convention, is simply written as M. paratuberculosis. Modern bacterial taxonomic methods have revised the name to Mycobacterium avium subspecies paratuberculosis (MAP).
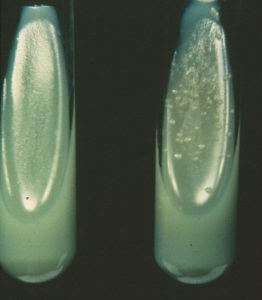 Twort had witnessed two unique biological characteristics of M. paratuberculosis that even today are the benchmarks for defining the organism: 1) extremely slow growth (8-12 weeks of incubation before seeing colonies in culture tubes), and 2) the fact that they require a substance known as mycobactin (provided by other mycobacteria such as M. phlei) to grow in laboratory culture media, a characteristic referred to as mycobactin-dependence. Laboratories commonly culture tissue or manure samples on tubes of laboratory culture media with and without mycobactin. Slow growth of acid-fast bacteria on culture media with mycobactin (right-hand tube) and not on media without mycobactin (left-hand tube in the photo to the right) is strong evidence for isolation of M. paratuberculosis. Today, microbiologists can go one step further and test for the presence of genetic elements unique to M. paratuberculosis. These genetic markers are given seemingly arcane designations like IS900, HspX, Mav2, F57, and 251F.
Twort had witnessed two unique biological characteristics of M. paratuberculosis that even today are the benchmarks for defining the organism: 1) extremely slow growth (8-12 weeks of incubation before seeing colonies in culture tubes), and 2) the fact that they require a substance known as mycobactin (provided by other mycobacteria such as M. phlei) to grow in laboratory culture media, a characteristic referred to as mycobactin-dependence. Laboratories commonly culture tissue or manure samples on tubes of laboratory culture media with and without mycobactin. Slow growth of acid-fast bacteria on culture media with mycobactin (right-hand tube) and not on media without mycobactin (left-hand tube in the photo to the right) is strong evidence for isolation of M. paratuberculosis. Today, microbiologists can go one step further and test for the presence of genetic elements unique to M. paratuberculosis. These genetic markers are given seemingly arcane designations like IS900, HspX, Mav2, F57, and 251F.
Early warnings
Leading U.S. veterinarians realized early on that Johne’s disease was a serious problem. K.F. Meyer, in a 1913 report to the Pennsylvania State Livestock Sanitary Board, said:
“….the economic loss will become one of a very serious nature if necessary steps for the control of this disease, which has been brought to this country by importation, are not taken.”
Bulletin 343 from the University of Wisconsin Agricultural Experiment Station, published in 1922, provided a 22 page description of Johne’s disease, complete with pictures of affected animals, damaged tissues and laboratory cultures of M. paratuberculosis. In this publication, these astute scientists gave sound advice to farmers, advice that is still true today, such as:
“…eradication (of the infection from a herd) by elimination of those animals showing symptoms would, in most instances, not be successful.”
“Prompt removal of all suspicious animals from the herd and care in the purchase of animals will certainly do much to limit the continued spread of this disease.”
“The prompt separation of calf and dam will, undoubtedly, prevent the infection of the former, as it does in tuberculosis.”
More warnings
In 1924 Drs. Larson, Beach and Wisnicky from the University of Wisconsin reported on the occurrence of Johne’s disease in Wisconsin dairy herds in the Journal of the American Veterinary Medical Association. They also offered this advice:
“The disease has, at present, a limited number of sources from which it can spread …..These sources of infection will continually increase, unless agencies are operative to offset the constantly increasing commerce in cattle.”
This same article quoted other experts as saying:
“…the present position of Johne’s disease is compared with that of bovine tuberculosis 60 years ago and, if not controlled, it may become a more troublesome scourge for future generations than tuberculosis is for the present generation of cattle owners.”
Despite the seriousness of the disease and the cogency of the scientific warnings, it took another seventy years before any country developed systematic programs to stop the spread of paratuberculosis among cattle herds.
The dairy industry in the U.S. and many other countries continued growing. In order to increase efficiency, dairy herds became larger. Cattle were purchased and traded without regard for whether they were infected with M. paratuberculosis or whether they came from an infected herd. What was once a rare disease of dairy cattle soon became common-place.
Today’s veterinarians routinely hear farmers say the same thing Dr. Harms heard over 110 years ago:

“Hey Doc! While you’re here, would you please look at Bossie or Elsie or Gertrude? She had a nice calf about three weeks ago and has been eating well, but she looks too thin, isn’t milking as well as she should. She also seems to have rather loose manure.”
Insidious and ignored
Johne’s disease typically shows up as a curious combination of weight loss and decreasing milk production in cows with a healthy appetite and no fever. Other than being thinner than their herd-mates, these cows typically don’t look or act sick. Of course, a dairy cow that’s not giving her fair share of milk is not destined to stay around long. Most farmers simply send her to slaughter and replace her with another, better-producing cow.
Johne’s disease has continued spreading among herds, between states and regions, and between countries for a multitude of reasons. First, the cattle industry failed to heed the warnings of veterinarians from the 1920s. Second, the disease spreads insidiously and the clinical signs are rather subtle, hence easily overlooked or ignored. Third, national and international veterinary regulations have either been too lax or too often ignored. Fourth, the economic impact of the disease is subtle and not readily apparent until a high proportion of the cows in a herd are infected.

As a consequence, over 50% of dairy herds in most major dairy producing countries are now MAP-infected. The official estimate in the U.S. from a survey conducted in 2007 and published in 2013 is that 91% of U.S. dairy herds are infected. This is up from USDA’s 1996 estimate that 21.6% of U.S. dairy herds have paratuberculosis. The infection has spilled over into a variety of other animal species, including but not limited to; beef cattle, sheep, goats, bison, deer, elk, llamas, alpacas, camels, antelope and other exotic ruminants that can be found in zoos. There are also two reports of nonhuman primates having paratuberculosis, one in Rhesus macaques and the other in a Mandrill baboon. Multiple species of wildlife also have been found to be infected but in most instances these animals do not develop progressive disease or pass the MAP organisms in their feces and so are considered a “dead-end” host. This link takes you to a page on this website describing the many non-ruminant animals that have been found to be MAP-infected.
The natural history of Johne’s disease
The natural host for MAP is ruminants, meaning animals with a four-chambered stomach that chew their cud as they digest their diet of grass and hay. A natural host is one in which the relationship between host and microbe is relatively well-balanced. The host does not mount an immune response that ejects the microbe from the body and the microbe does not kill the host – at least not for a long time. The causes of TB in humans and cattle, and the cause of Johne’s disease, M. paratuberculosis (MAP), are excellent examples of this balanced host-pathogen relationship. These situations allow MAP to silently multiply and leave one host via milk and manure to set up residence in another host, usually a newborn, after being consumed.
Owners accidentally introduce MAP to their herds or flocks by mistakenly buying an infected animal. This happens because they do not know much about Johne’s disease or because they can’t find herds proven to be free of MAP infection, or simply don’t do their microbial homework. Movement of MAP-infected animals into non-infected herds is how Johne’s disease spread from Europe to the U.S. and how it continues infecting countries such as China that are developing their dairy or other animal agriculture industries. International regulations promulgated by the World Organization for Animal Health (O.I.E.) are woefully inadequate at blocking movement of MAP-infected animals. Rules to help limit the chances of spread of MAP infections within some countries, notably Australia, are far more effective, but they are not perfect. For more than a decade, the USDA has had a system for certifying herds with the least risk of being MAP-infected, but few herd owners see a sound economic reason for participating. Consequently, the MAP/Johne’s epidemic continues expanding to encompass more and more herds in more and more locations around the world.

Newborn animals are most susceptible to MAP infection, which they get by ingesting the organism. In dairy calves, this can happen in a variety of ways. Often calves are born in a large maternity pen occupied by a number of pregnant cows on the verge of giving birth. Naturally, the pen is kept as clean as possible. However, cows produce about 60 pounds of manure a day and it can be challenging to keep things clean. Shortly after its birth, the newborn calf will try to stand and find its mother’s teat for its first crucial drink of colostrum, rich in antibodies and nutrients. However, the calf will typically fall on its face as it tries to get those wobbly legs to work. And sometimes, not surprisingly, it does a face-plant right into a cow pie! Once on its feet, the calf tries to home in on mom’s udder. Its efforts are not always on target and the first few efforts to suckle may be on mom’s back leg or some other part of the cow. Eventually, the calf will successfully find the teat to which it latches and begins to suckle, just like a newborn baby. Of course, the teat surface – not to mention the mother cow’s hind leg – has in contact with the bedding in the maternity pen and thus well may have also been in contact with manure from any of the cows in the pen that day. So, in these first few hours after being born, a calf has multiple opportunities to swallow a bit of MAP-laden manure.
Ingestion of MAP-contaminated manure is probably the most common means of exposure and infection for newborn calves. However, in cows that are in the more advanced stages of infection, the MAP bacteria escape from the infected intestinal wall and local lymph node, and are carried throughout the cow’s blood stream. The cow’s natural defenses will try to clear these bacteria from the blood, causing them to concentrate in the liver and spleen, organs that filter blood to remove microbes and aging red blood cells. But MAP will be found not only in the liver and spleen. It also will cross into the udder and then be found in the milk. And, if that cow is pregnant, the MAP bacteria in the blood will cross the placenta and infect the fetus resulting in a calf that is MAP-infected even before birth.
 It is vital that calves drink three or four quarts of colostrum within six hours of birth. This is the only means by which they can get antibodies to protect them from viral and bacterial pathogens they will inevitably encounter during their first weeks of life. After their colostrum meal, calves are fed milk until they are weaned at 8-12 weeks of age. Many modern dairy farms will feed a product called “milk replacer.” This is essentially pasteurized milk in powder form, just like human infant formula. However, on some farms raw milk, called “waste milk,” is fed to calves. This milk is of a quality not suitable for human consumption. It comes from cows with mastitis, which causes the milk to contain too many white blood cells, or it is derived from cows that have been treated with antibiotics and thus is unsuitable for human consumption until after the drug has been cleared from the animal’s system. Raw (not heat-treated) waste milk can harbor a variety of microbial pathogens. MAP is one of them.
It is vital that calves drink three or four quarts of colostrum within six hours of birth. This is the only means by which they can get antibodies to protect them from viral and bacterial pathogens they will inevitably encounter during their first weeks of life. After their colostrum meal, calves are fed milk until they are weaned at 8-12 weeks of age. Many modern dairy farms will feed a product called “milk replacer.” This is essentially pasteurized milk in powder form, just like human infant formula. However, on some farms raw milk, called “waste milk,” is fed to calves. This milk is of a quality not suitable for human consumption. It comes from cows with mastitis, which causes the milk to contain too many white blood cells, or it is derived from cows that have been treated with antibiotics and thus is unsuitable for human consumption until after the drug has been cleared from the animal’s system. Raw (not heat-treated) waste milk can harbor a variety of microbial pathogens. MAP is one of them.
MAP has cleverly devised a way to efficiently hop from infected adult cows to their calves. Other cattle pathogens like bovine brucellosis have adopted similar strategies. Indeed, there is an association between the MAP infectious status of a cow and her calves. This is truer in animal husbandry systems such as for beef cattle, where a calf stays at its mother’s side nursing until it is six months old or more. This association is weaker in husbandry systems like that on most modern dairy farms, where the calf is removed from its mother soon after birth, reared well away from adult cows and fed milk artificially.
In cattle, the time lag between initial infection as a fetus or neonate until clinical signs of Johne’s disease and death can be as short as 2 years or as long as 12, or even more. We don’t really know how long cattle could survive with Johne’s disease because farmers simply send them to slaughter when they start going downhill, before they die a “natural” death. The speed with which the infection progresses is governed mostly by the dose of MAP to which the calf was exposed. It may also be affected by the age of exposure or the genetics of the animal. Geneticists have found evidence that some cattle are more resistant to the infection than others. None are totally resistant, however. For most of this prolonged incubation period, before an animal shows visible signs of Johne’s disease, MAP will be found in the wall of the intestine, in manure and in milk, although the abundance in milk is much lower than in manure.
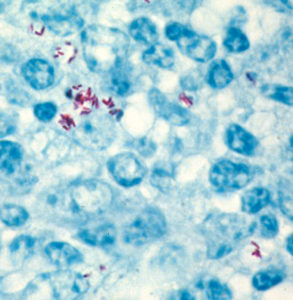 This story of the pathogenesis of Johne’s disease is similar for most animals that are considered its natural host, namely ruminants. In ruminants MAP has adopted a life style as an “obligate parasite.” Although its ancestors were able to grow and replicate in soil and water, MAP has evolved along its own special track, adopting ruminant animals (specifically inside certain “white blood cells” called macrophages – blue cells in the adjacent photo. The MAP bacteria are stained red) as its host. In fact, it has lost the ability to replicate outside animals. It is obligated to a life inside the cells of its host; it is therefore called an obligate intracellular parasite.
This story of the pathogenesis of Johne’s disease is similar for most animals that are considered its natural host, namely ruminants. In ruminants MAP has adopted a life style as an “obligate parasite.” Although its ancestors were able to grow and replicate in soil and water, MAP has evolved along its own special track, adopting ruminant animals (specifically inside certain “white blood cells” called macrophages – blue cells in the adjacent photo. The MAP bacteria are stained red) as its host. In fact, it has lost the ability to replicate outside animals. It is obligated to a life inside the cells of its host; it is therefore called an obligate intracellular parasite.
Once MAP leaves its warm, nutritious intracellular home, it must wait patiently (it has no in dependent means of moving) until it is eaten by a susceptible host animal. Then, the infection and replication process begins again. Since this wait can be a very long one (imagine MAP sitting patiently, biding its time in a cow pie out in the middle of a pasture), MAP has evolved strategies for resistance to environmental conditions like heat, freezing, drying, and sunlight: factors that effectively kill most microbes. Under adverse conditions, MAP changes into a resting, or dormant form of bacterial cell called a spore. Bacterial spores are notoriously resistant to both physical and chemical factors that kill ordinary bacteria.
The blame game
MAP is an equal opportunity infection. It attacks cattle of all breeds on farms of all sizes and types. And before anyone jumps to conclusions, this is not a problem caused by so called “factory farms”. In fact, it is the large farms that have the ability to most readily implement measures to control the infection. Specifically, they can afford to have farm workers monitor maternity pens 24/7 and remove calves from the source of infection immediately after birth. For 50 cow mom and pop operations there is nobody there to attend a calving that happens at 2:00 AM. Large farms also routinely use milk replacer or install pasteurizers to prepare milk for feeding calves. Smaller farms tend to use waste milk to save money. Surely organic farms are free of Johne’s disease! Guess again. Organic dairies are just as likely to have a Johne’s disease problem as any other. Organic regulations pertain only to chemical and antibiotic residues, not to the health of the animals.
Why not treat animals with Johne’s disease?
Treatment of Johne’s disease in cattle has been attempted, but without much success. Although it may be theoretically possible to cure this infection, the antibiotics required are not legal for use in food-producing animals. Hence all the meat and milk from animals treated with such drugs would have to be discarded, not eaten. Moreover should anyone decide to attempt this, the drugs are expensive: dosages for a 1200 pound animal are large, and the duration of treatment must be 12 months or longer. Consequently, such a treatment regimen, if attempted, would cost 50 times the value of most dairy cattle, which simply does not make economic sense. So, animals that develop signs of Johne’s disease or are found positive by diagnostic tests are simply sent to slaughter. There is no small irony here: Even if the cost of treating Johne’s cattle were low enough to be economically feasible, regulations against human consumption of the relevant antibiotics would preclude doing so … and as a result, untreated cattle, ripe with untreated and virulent MAP bacteria, are made into meat – mostly hamburger – for direct human consumption!
Recently, some investigators have claimed to be able to prevent or even cure Johne’s disease with live cultures of a bacterium known as Deitzia – a “yogurt-like” probiotic approach. This work, while promising and potentially helpful to humans with Crohn’s disease, has yet to be reproduced in a carefully controlled trial by scientists independent of the company selling the product.
How about vaccination?
There are vaccines for Johne’s disease, but they are not very effective. In fact, there are no effective vaccines for any bacteria in the genus Mycobacterium, including those causing tuberculosis and leprosy. Mankind has been pursuing vaccines for mycobacterial infections without success since Robert Koch first described Mycobacterium tuberculosis in 1882. Scientists the world over continue trying.
There is only one USDA-licensed vaccine for Johne’s disease in the U.S., known as Mycopar™. Its use is tightly regulated because vaccination with Mycopar™ causes animals to test positive for bovine tuberculosis, which seriously complicates U.S. efforts to eradicate bovine TB. The most recent clinical trial of Mycopar™ in dairy herds with Johne’s disease was published in March 2013. In that case, every other calf born on three Wisconsin dairy farms was vaccinated with Mycopar™ before they reached 35 days old; they were then followed for several years. The authors reported that when vaccinated and non-vaccinated animals were compared, there was no significant difference in the rate of clinical Johne’s disease, no improvement in the longevity of cows in the herd, and no difference in milk production or reproduction. They did, however, observe that the vaccinated cows had a lower frequency of detectable MAP in their feces.
 Although the Mycopar™ vaccine may somewhat lower the frequency of clinical Johne’s disease, many vaccinated cows still progress to this end stage of the infection. Pictured here is a cow that had been Johne’s-vaccinated and yet still developed all of the classical signs of Johne’s disease and the diagnosis was confirmed by detecting MAP in her feces. Notice the golf ball-size lumps in this cows brisket caused by the vaccine administered to this animal when it was less than 35 days old.
Although the Mycopar™ vaccine may somewhat lower the frequency of clinical Johne’s disease, many vaccinated cows still progress to this end stage of the infection. Pictured here is a cow that had been Johne’s-vaccinated and yet still developed all of the classical signs of Johne’s disease and the diagnosis was confirmed by detecting MAP in her feces. Notice the golf ball-size lumps in this cows brisket caused by the vaccine administered to this animal when it was less than 35 days old.
 In addition to interfering with the bovine TB eradication program, vaccines for Johne’s disease often cause significant damage at the site of inoculation, the brisket. These “vaccine-induced granulomas” range in size from golf balls to soccer balls. They tend to get banged around as the animal approaches a feed bunk and can get infected and ooze pus.
In addition to interfering with the bovine TB eradication program, vaccines for Johne’s disease often cause significant damage at the site of inoculation, the brisket. These “vaccine-induced granulomas” range in size from golf balls to soccer balls. They tend to get banged around as the animal approaches a feed bunk and can get infected and ooze pus.
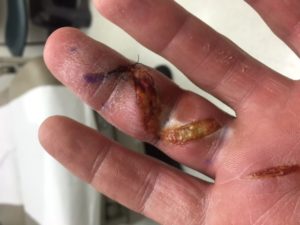 Animals are not the only ones that get vaccinated on the farm. Occasionally, when struggling with a rambunctious calf while trying to vaccinate it, animal holders or veterinarians will accidentally get stuck with a needle. Even the smallest prick from a needle that has Mycopar on it will lead to a painful swelling. This has happened often enough to warrant publications about these events, since the human pathology that results are typically quite graphic. For veterinarians, the painful lesions – that may take months or longer to resolve – can be incapacitating.
Animals are not the only ones that get vaccinated on the farm. Occasionally, when struggling with a rambunctious calf while trying to vaccinate it, animal holders or veterinarians will accidentally get stuck with a needle. Even the smallest prick from a needle that has Mycopar on it will lead to a painful swelling. This has happened often enough to warrant publications about these events, since the human pathology that results are typically quite graphic. For veterinarians, the painful lesions – that may take months or longer to resolve – can be incapacitating.
In spite of the shortcomings of vaccination, this control strategy seems the only affordable option for sheep and goat owners. The Gudair® vaccine (sold by Zoetis, a sponsor of this website) is widely used in Australia and Spain for sheep and goats.
Then what CAN be done?
Control of Johne’s disease in farm animals is fairly straight-forward, but requires consistency and lots of patience. To significantly reduce the infection rate in a dairy herd, herd managers must follow recommended changes in management, specifically designed to limit transfer of the MAP infection from cows to calves. The most critical changes for dairy herds are:
- Only allow MAP test-negative cows into the main maternity pen,
- Remove the newborn calf and cow from that pen within an hour of birth,
- Feed the calf clean colostrum from a MAP test-negative cow, and then
- Feed the calf pasteurized milk, meaning milk replacer or milk from a dairy herd that is pasteurized on the farm.
To find sources of MAP, herd owners must test every cow in the herd at least once during each lactation (basically once a year) and they must consistently act on the test results by culling (slaughtering) or segregating the test-positive animals. And, most importantly, they must do this consistently for a period of at least 6 or more years. Published evidence shows that such a program is very successful at reducing the infection rate in dairy herds. The strategies in other species are similar in concept but must be adapted to different animal husbandry methods.
For more specifics about prevention and control of Johne’s disease in specific kinds of animals look under “Animal Type” for information regarding beef cattle, goats, sheep, deer and elk, bison, water buffalo, wild ruminants, zoo ruminants, and other non-ruminant animals.
Animals become or produce food
If you are a consumer, particularly if you are not a vegan, you may be thinking: “Hey! What happens to these animals with Johne’s disease that are sent to slaughter? And, where does their milk go?” The troublesome truth is that every day, dairy cattle, beef cattle, goats and sheep with Johne’s disease are sent to slaughter because they are no longer healthy productive animals. Veterinary inspectors who review the condition of live animals recognize that they may be a bit thin, but are otherwise healthy and are therefore deemed acceptable for use as food. On the slaughterhouse floor, veterinary inspectors who examine the insides of each carcass report seeing intestinal pathology indicative of Johne’s disease on a regular basis, but they see nothing that by law requires carcass condemnation (as would be the case for pathology indicative, for example, of bovine TB). Thus, all these infected animals enter our food supply. Most become ground beef.

Likewise, dairy animals with Johne’s disease – be they dairy cattle, goats or sheep – produce milk that enters the food supply. Most milk ends up as some form of cheese. The production of many but not all cheeses involves pasteurization of the milk as a first step in the production process. However, some cheeses can only be made from raw milk. By law, virtually all milk used for fluid consumption in developed countries is pasteurized. However, some “health food” advocates in the U.S. stridently argue that raw milk is better for you and have lobbied to change laws prohibiting sale of raw milk or have found other creative ways to circumvent pasteurization laws. Many of the raw milk producers and likely all of the raw milk consumers are unaware of the potential risks of drinking MAP. They seldom if ever ask if the animals producing their milk have Johne’s disease. If the herd providing raw milk does have Johne’s, then the milk is highly likely to be laced with MAP along with a long list of other zoonotic pathogens.
It is controversial whether MAP is killed by pasteurization or other food manufacturing practices. Suffice it to say that live MAP have been recovered form retail pasteurized milk in the U.S., U.K. Czech Republic, Argentina, Brazil and India.
This link provides much more information about MAP in food.
This link provides more about the potential human health consequences (zoonotic potential) of MAP.
Why aren’t producers doing something about this?
MAP is a nagging but not very economically important problem in most herds and flocks of food-producing animals. As businesses with narrow profit margins, herd owners are not motivated to spend money to fix a problem that does not seem to be economically crucial. A common complaint of dairy herd owners is that a Johne’s disease control program is more costly than the disease, a variant on “If it ain’t broke, don’t fix it” – “if it costs too much, don’t bother.”
Many countries have launched national programs to control Johne’s disease. When government agencies subsidize the costs of Johne’s disease control, more producers participate in control and herd certification programs. When the money disappears, they stop. As a result, MAP flows, quite literally, through the food chain to land on the consumer’s plate as MAP-burgers, or in their glass of milk. Even scarier is the potential that MAP is in infant formula where, even if dead, it may trigger pathology. Since medical science does not currently label MAP a zoonotic pathogen – meaning an infectious disease transmitted from animal to humans – there is no legal way to stop MAP from contaminating foods. So, who decides if MAP is zoonotic? Good question!
This link provides access to URLs for national programs and websites concerning MAP.
The relationship between MAP, food-producing animals and humans is complex and still somewhat murky. Solutions will require more scientific investigation, and a rational dialogue based on mutual respect between food producers in animal agriculture, veterinarians, government agencies and medical doctors. Among these voices, consumers have been notably silent. The Johnes.org website is one step toward changing this situation.
Check out the NEWS page for the latest developments of interest to animal owners and veterinarians. Click on the “subscribe” button if you want to be notified by email when news items are posted.
Click here for a PDF of this full article.