This page leads readers to publications documenting MAP infections in nonruminants. Photos are included merely to demonstrate the diversity of affected animals. In two instances, a human and a pot-bellied pig, the actual MAP-infected “animal” is shown.
Humans (Homo sapiens)

One man’s story – meet Dr.med. vet. Heinrich Dahmen.
Until November 2000 I was perfectly well. Then, I began to notice an increasing stool frequency. Early in 2001 I developed abdominal pain and mouth ulcers and noticed that I was suffering from lack of energy. In April 2001 I also noticed bloody stools. A colonoscopy showed an inflammation of the left colon consistent with Crohn’s disease.
My doctor wanted to put me on mesalazine, a common anti-inflammatory drug at that time, but I refused. I had a vague memory of a possible link between Crohn’s disease and Johne’s disease. A friend of mine, Prof. Wolfgang Klee from the veterinary school at the University of Munich gave me Prof. M. Collins phone number and in May 2001 I called him. Dr. Collins referred me to Prof. John Hermon-Taylor in London.
In London, I had a second ileocolonoscopy at London Bridge Hospital on June 20, 2001, performed by Dr. Jeremy Sanderson. This exam found aphthous ulcers and colitis in the recto-sigmoid region. Mucosal biopsies were obtained for PCR and culture testing for MAP. Serum was obtained to examine for antibodies against selected MAP proteins. All three biopsies from the inflamed sigmoid colon and rectum were PCR-positive for MAP. This was supported by the additional finding that I had elevated IgG antibodies against recombinant AhpC antigen of MAP.
I was treated for two years with 150 mg rifabutin and 250 mg clarithromycin in the morning and 150 mg rifabutin and 500 mg clarithromycin in the evening. Beginning in November 2001, I also took 50 mg clofazimine once a day.
After two or three weeks I had a mild Jarisch-Herxheimer reaction due to the destruction of MAP and release of its antigens. Thus, all medications were stopped. After a week, I started the initial therapy again starting with low antibiotic doses which gradually increased to the full dosages.
By late fall 2001 I felt perfectly well again, and all the symptoms of Crohn’s disease had gone. However, on Dr. Hermon-Taylor’s advice I continued the medication until August 2003. A follow-up colonoscopy was carried out on September 24, 2003 at London Bridge Hospital, again by Dr. J. Sanderson. Again, tissue samples were collected. The results of the colonoscopy and histology were “normal gut”. The IS900 Nested PCRs on all biopsies were negative too. I was healed!!
It’s now 15 years later (fall 2018) and I´m still in good shape and continuing to practice veterinary medicine in Germany, thanks to the great work of Prof. John Hermon-Taylor.
How did I acquire a MAP infection? There are two possibilities: 1) I consumed raw milk over long periods during my childhood, and/or 2) I am regularly exposed cattle with Johne’s disease in my large animal practice.
Publications:
A similar story published as a letter to the editor: Chamberlin, et al. Successful treatment of Crohn’s disease patient infected with bacteremic Mycobacterium paratuberculosis. American Journal of Gastroenterology, 102(3): 689-691, 2007.
A comprehensive evaluation of 28 case-control studies where Crohn’s patients and control subjects were tested for MAP infection by PCR or ELISA finding strong evidence of an association by both diagnostic methods: Feller et al. 2007. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infectious Disease, 7:607-613.
A second, independent, meta-analysis that corroborating the findings of the Feller report: Abubakar, et al. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-based techniques: A systemic review and meta-analysis. Inflammatory Bowel Diseases 14(3):401-410, 2008.
See “Zoonotic Potential” section of this website for more information.
Dog (Canis lupus familiaris)

Publications:
Background (excerpt from the Glanemann article): This study was conducted to examine intestinal biopsies from dogs with and without a history of chronic diarrhea or vomiting or both for the presence of MAP-specific DNA using a nested IS900 PCR, a seminested f57 PCR, and a recently developed triplex real-time PCR (ISMav2, F57, & internal amplification control) to determine whether the detection of MAP-DNA is associated with a specific histopathologic diagnosis in dogs with chronic gastrointestinal disease.
Findings (excerpt from abstract): Histopathology of the biopsies was indicative of inflammatory bowel disease (IBD) in 17 and neoplasia in 6 dogs. Six dogs showing nonspecific changes responded to diet and were classified as having food-responsive enteropathy. In 13 dogs a final diagnosis was not established. MAP-specific DNA was detected and confirmed by sequencing in 8 dogs (19%). These dogs were diagnosed with food-responsive enteropathy (n=3), IBD (n=2), and open diagnosis (n=3). MAP-specific DNA was not detected in dogs with no gastrointestinal disease.
Conclusions and clinical importance: MAP-specific DNA was detected in approximately one fifth of dogs with chronic gastrointestinal disease and might play a role as a pathogenic agent. Apart from animal welfare, the zoonotic aspect warrants further studies addressing the viability of MAP organism in canine intestinal biopsies by culture.
Rhesus macaque (Macaca mulatta)

Publication: H.M. McClure et al. Mycobacterium paratuberculosis infection in a colony of stumptail macaques (Macaca arctoides). Journal of Infectious Diseases. 155(5):1011-9, May, 1987.
Abstract: Mycobacterium paratuberculosis infection was documented in a colony of stumptail macaque monkeys (Macaca arctoides), with 29 (76%) of 38 monkeys infected and shedding organisms in feces. Thirteen deaths have occurred during the past five years. Animals without overt clinical disease were shedding as many as 2 X 10(6) colony-forming units of M. paratuberculosis/g of feces. Intestinal tissues from animals dying of this disease contained up to 10(8) colony-forming units of M. paratuberculosis/g of tissue. The clinical and pathological features of paratuberculosis in this species were comparable to those reported for paratuberculosis in ruminants and Mycobacterium avium infections in primates. By enzyme-linked immunosorbent assay, antibodies to M. paratuberculosis were found in 79%-84% of the animals. Antibodies could not be detected in six animals with clinical disease. These findings extend the natural host range of M. paratuberculosis to include nonhuman primates and add support to current suggestions that M. paratuberculosis may be pathogenic for humans.
Mandrill (Papio sphinx)

Publication: L.S. Zwick et al. Paratuberculosis in a mandrill (Papio sphinx). Journal of Veterinary Diagnostic Investigation 14:326-328, 2002.
Abstract: A 2.5-year-old captive female mandrill (Papio sphinx) died following a protracted course of intermittent abdominal bloat, diarrhea, and severe weight loss. Necropsy revealed emaciation and marked gastrointestinal distention with gas and ingesta. Histologic evaluation revealed severe diffuse granulomatous enterocolitis and mesenteric lymphadenitis with massive numbers of 1–2-micrometer acid-fast bacilli within macrophages. Additionally, there was moderate to severe multifocal myocardial and vascular amyloidosis, moderate multifocal pyogranulomatous interstitial pneumonia with no acid-fast bacteria, and moderate multifocal glossal candidiasis. Samples of feces, ileum, and colon were positive for Mycobacterium avium subsp. paratuberculosis by radiometric culture and a polymerase chain reaction-amplified DNA probe specific for the insertion sequence IS900 of this organism.
Note (from Wikipedia): The mandrill (Mandrillus sphinx) is a primate of the Old World monkey (Cercopithecidae) family. It is one of two species assigned to the genus Mandrillus, along with the drill. Both the mandrill and the drill were once classified as baboons in the genus Papio, but they now have their own genus, Mandrillus. Although they look superficially like baboons, they are more closely related to Cercocebus mangabeys. Mandrills are found in southern Cameroon, Gabon, Equatorial Guinea, and Congo. Mandrills mostly live in tropical rainforests. They live in very large groups. Mandrills have an omnivorous diet consisting mostly of fruits and insects. Their mating season peaks in July to September, with a corresponding birth peak in December to April. Mandrills are the world’s largest monkeys.
Vietnamese Pot-Bellied Pig (I pig)

Publication: L.R. Hancock et al. Natural transmission of MAP from a mixed breed Boer goat to a Vietnamese Pot-Bellied Pig. Poster presented at the 14-ICP held in Mexico, June 2018. Interesting note: the first author was the owner of both the pig and goat and a new graduate from veterinary school.
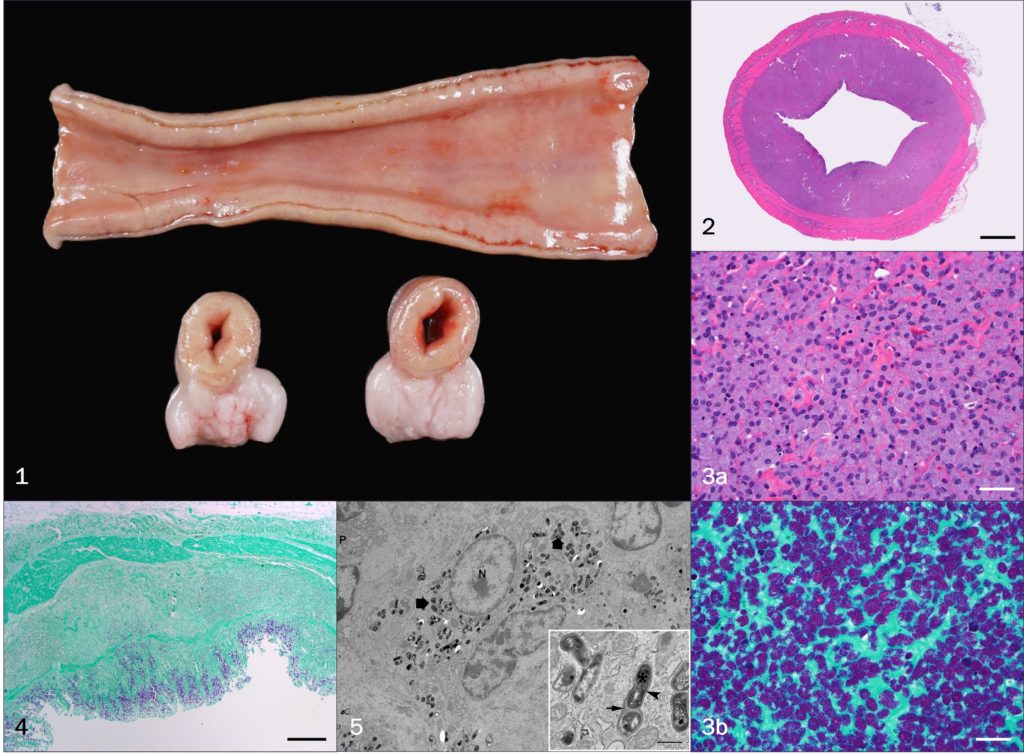
Abstract: A 12-year-old male neutered Vietnamese pot-bellied pig developed progressive inappetence, lethargy and diarrhea. The physical exam showed normal body condition, normal gastrointestinal sounds, and no tenderness on abdominal palpation. A complete blood count showed a marked increase in neutrophils (34.4 x 10(3)/uL). Serum chemistries indicated normal kidney and liver function. However, the pig had low total serum protein (TP) and albumin (A) values (TP 4.1 g/dL; A 1.5 g/dL). Abdominal ultrasonography revealed a prominent small intestine with thickened walls throughout the abdomen. Intestinal loops were filled but not distended and motility was reduced. This pet pig was a long-time companion to an adopted mixed breed Boer goat that died from paratuberculosis two and a half years prior to onset of the pig’s clinical signs (confirmed by histopathology and PCR). For this reason, and because of the ultrasound and serum chemistry findings, a fecal sample was submitted for Mycobacterium avium subsp. paratuberculosis (MAP) IS900 PCR. The PCR was positive with a Ct value of 20.8 (cut-off for a positive = 35) indicating the pig was a heavy shedder of MAP. The pig’s condition continued to decline despite therapy, and was euthanized. Necropsy and histopathology confirmed a diagnosis of paratuberculosis. Cultures recovered MAP. Whole genome sequencing showed that the MPA strain from the pig was closely related to the K10 strain of MAP, the first strain fully sequenced. The strain from the goat which died two years before the pig got sick was not saved for comparison. This represents the first report of paratuberculosis in a pot-bellied pig and the one of three reports of natural transmission of paratuberculosis to swine. This finding verifies that swine, in particular pot-bellied pigs, are susceptible to MAP and potentially provides a useful “small” animal model for study of a Crohn’s disease-like illness.
Pathology Images (by J.A. Dykstra):
Notes from Wikipedia: Vietnamese Pot-bellied is the exonym for the Lon I, simply I pig, an endangered traditional Vietnamese breed of small domestic pig.
The I is uniformly black and has short legs and a low-hanging belly, from which the name derives.The I is reared for meat. It is slow-growing, but the meat has good flavour. The I was depicted in the traditional Đông Hồ paintings of Bắc Ninh province as a symbol of happiness, satiety and wealth.
This is a traditional Vietnamese breed is thought to have originated in Nam Định province of Vietnam, in the Red River Delta. Until the 1970s the I was probably the most numerous pig breed in northern Vietnam, with numbers running into millions. From that time, the more productive Móng Cái began to supplant it. The National Institute of Animal Husbandry of Vietnam started a conservation programme, with subsidies for farmers who reared purebred stock, but this had little benefit – there was some increase in numbers, but at the cost of increased inbreeding. In 1991, the total population of the I was estimated at 675,000, and by 2010 the estimated number was 120. In 2003 the National Institute of Animal Husbandry listed its conservation status as “critical”; in 2007 the FAO listed it as “endangered”.
Small numbers of I pigs were exported in the 1960s to Canada and Sweden, to be kept in zoos or to be used for laboratory experiments. Within a decade, the I had spread to animal parks in other countries in Europe; a few were reared on smallholdings. The I entered the United States from Canada in the mid-1980s, and by the end of the decade the “pot-bellied pig” was being marketed as a pet. Not all of these were purebred, and some grew to considerable size; the fad was short-lived.
Sicilian Ass

Abstract: A presumptive diagnosis of paratuberculosis was made in a Sicilian ass on the basis of a history of chronic diarrhea and weight loss, pasture exposure to a heifer with paratuberculosis confirmed by bacterial culture of feces, postmortem identification of granulomatous inflammation of the intestine containing acid-fast organisms, the absence of acid-fast organisms in extra-enteric tissues, and the absence of exposure to tuberculosis. The literature on paratuberculosis in equids is reviewed. The potential for cross-species transmission is emphasized. Justification for consideration of Mycobacterium paratuberculosis infection in the differential diagnoses of equine granulomatous enteritis is discussed.
Wallaby (Macropus sp.)

Background (excerpt from publication): Kangaroo Island (KI) in South Australia provided an ideal environment in which to further examine the possible role of macropods in the epidemiology of OJD, because large numbers of Western grey kangaroos (Macropus fuliginosus fuliginosus) and Tammar wallabies (Macropus eugenii decres) grazed alongside infected sheep on most KI properties at the time of this study in 2001 and 2002. Macropods might simply act as mechanical vectors, passively excreting M. a. paratuberculosis that survive passage through the gut, and thus provide a means of spreading the organism from infected to uninfected flocks. Alternatively, they could become infected themselves and develop lesions of paratuberculosis, potentially amplifying the organism, and become a wildlife reservoir of infection. Not only would this facilitate the spread of M. a. paratuberculosis, it would seriously hamper any efforts at eradication. In this study we aimed to detect and estimate prevalence of infection of macropods with M. a. paratuberculosis on infected sheep farms, and to investigate pathological changes associated with that infection.
Abstract: The aim of this study was to determine whether Mycobacterium avium subspecies paratuberculosis (M. a. paratuberculosis) infection was present in macropods grazing with infected sheep on Kangaroo Island in 2001–2002, and to assess the likely role of such infection in the epidemiology of ovine paratuberculosis. Ileum and associated lymphatics from 482 macropods were examined using radiometric culture followed by PCR for IS900 and restriction endonuclease analysis (REA) for species identification, and isolates were strain typed using PCR for IS1311 and REA. Ileum and mesenteric lymph nodes from animals with positive tissue cultures or gross lesions suggestive of paratuberculosis were examined histologically. Faeces from a total of 840 animals were cultured in pools of 20, and individual faecal cultures were done from tissue culture positive animals, from those with microscopic lesions, and from selected animals with gross lesions. Eight animals (1.7%) yielded positive tissue cultures, and all isolates were the sheep (S) strain. Two animals that were tissue culture positive also had histopathological evidence of paratuberculosis. Twelve culture negative animals had microscopic lesions consistent with mycobacterial infection, and M. genavense was identified by PCR from a paraffin block from one of these animals. All faecal cultures were negative. These results indicate that a small proportion of macropods can become infected with M. a. paratuberculosis when grazing with infected sheep. However, excretion of large numbers of viable organisms is rare in macropods, and it is unlikely that macropods provide a wildlife reservoir of infection that would seriously compromise control efforts for paratuberculosis in sheep.
European Wild Rabbit (Oryctolagus cuniculus)

Background (excerpt from publication): The known wildlife host-range for paratuberculosis has extended over recent years to include a number of non-ruminant species (Beard et al., 2001a) including the European rabbit (Oryctolagus cuniculus) (Greig et al., 1997). Rabbits are sub-clinical carriers of MAP infection (Beard et al., 2001c), and can shed up to 7 × 10(5) colony forming units (CFU)/g faeces (Daniels et al., 2003a), with on-farm prevalence rates of up to 53% reported among wild populations in the UK (Greig et al., 1999). Isolation of MAP from a range of rabbit tissues and excreta suggest the potential for horizontal, vertical and pseudo-vertical routes of transmission (Judge et al., 2006). These routes are estimated to be sufficient for the persistence of MAP infection in rabbit populations and suggest they may act as a wildlife reservoir for paratuberculosis i.e. the disease persists in the wildlife population in the absence of external sources of infection (Judge et al., 2007).
Abstract: There is increasing evidence that the European wild rabbit (Oryctolagus cuniculus) is a wildlife reservoir for paratuberculosis and infected populations may contribute to the persistence of infection in livestock. The aim of this study was to test the hypothesis that farms with difficulties controlling paratuberculosis in their cattle herds have a higher prevalence of Mycobacterium avium subsp. paratuberculosis (MAP) infection in their rabbit populations. A total of 281 rabbits from 13 beef farms in the East of Scotland were randomly sampled in early spring 2007. Participating farms were in paratuberculosis control programmes under the Premium Cattle Health Scheme (PCHS), and were classified as ‘responder’ (paratuberculosis under control) or ‘low responder’ (a persistent number of paratuberculosis-positive cattle despite control measures in place) farms.
Of the rabbits sampled, 23.8% tested positive for MAP, with those on ‘low responder’ farms having a greater probability of being infected (0.4) relative to rabbits on ‘responder’ farms (0.1). The association suggests that MAP-infected rabbits may contribute to the persistence of paratuberculosis in domestic livestock and undermine control strategies that focus on livestock alone. This study provides the first evidence of an association between the persistence of paratuberculosis in livestock despite the implementation of disease control strategies, and MAP-infected sympatric wild rabbit populations.
European Hare (Lepus europaeus ) – in Chile

Publication: M. Salgado, et al. European Hares in Chile: A different lagomorph reservoir for
Mycobacterium avium subsp. paratuberculosis? Journal of Wildlife Diseases, 47(3):734–738, 2011.
Background (excerpt from publication): To expand our understanding of potential nonruminant Map reservoirs, we wished to investigate a population of a different species of Lagomorpha, especially since the extensive infection and ensuing disease seen in Scottish rabbits have not been reported elsewhere. There are distinct ecologic and behavioral differences between the European rabbit and the European hare (Lepus europaeus) that could affect exposure to Map deposited by infected cattle into the environment. The hare’s offspring are precocial, while the rabbit’s are altricial, and hares are primarily solitary whereas rabbits form colonies congregating underground in burrows, resulting in young rabbits’ repeated exposure to feces from numerous adults (Broekhuizen and Maaskamp, 1982; Zorner, 1996). To augment our understanding of the susceptibility to and maintenance of Map infection in Lagomorpha, we conducted an investigation of a population of European hares sharing habitat with Map-infected dairy cattle in Chile.
Abstract: Ruminants are the principal host for infection by Mycobacterium avium subsp. paratuberculosis (Map), the cause of Johne’s disease. Based on studies of a Map-infected population of European rabbits Oryctolagus cuniculus) in Scotland, lagomorphs as a broad taxonomic order were proposed as potential nonruminant reservoirs for Map. To determine whether a different lagomorph species may serve as a wildlife reservoir, we investigated Map infection in European hares (Lepus europaeus) sharing habitat with known Map-infecteddairy cattle in southern Chile. Fecal, mesenteric lymph node, and ileal samples were aseptically collected from 385 wild hares for liquid culture and real-time polymerase chain reaction identification of acid-fast isolates. All tissue samples were also acid-fast stained and examined microscopically. We isolated Map from at least one tissue from 48 hares (12.6%) and fecal samples from 16 hares (4.2%). No Map was found in tissues of eight of the fecal culture–positive hares. Histologically, all tissues from all hares were within normal limits, and no acid-fast organisms were observed in any sample. Active infection, implying amplification of the organism secondary to resultant disease, was not evident. With this report Map isolations on a population versus incidental detection have now been made from two lagomorph species. However, although the rabbit population studied in Scotland appears to function as a Map reservoir, the hares studied in Chile appear to be a dead-end host, serving only as potential mechanical vectors for the organism.

