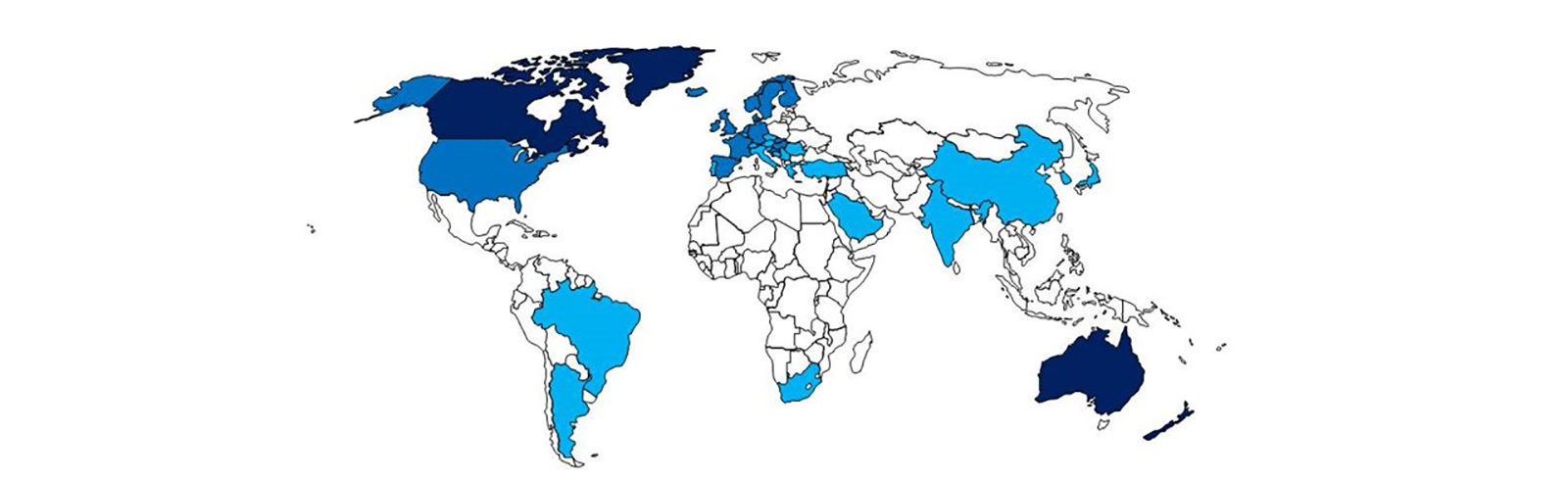
The graphic above depicts the incidence of Crohn’s disease by country (darker blue = higher incidence). It is adapted from the 2013 publication by Ng et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut oi:10.1136/gutjnl-2012-303661. It mirrors the map for prevalence of Johne’s disease by country that can be found on the Dairy/Epidemiology page.
Whether MAP is a zoonotic pathogen (infection of animals transmissible to humans) is the single most critical scientific question regarding this pathogen. The answer will drive every facet of the diagnosis, control and prevention of this infection in animals.
If not a zoonosis, then Johne’s disease is a bothersome, but not overly important, animal health issue.
On the other hand, if it is a zoonosis, then MAP affects animals and humans alike, and animals are the source of human infections and disease. Thus, it would demand more aggressive control of MAP in food-producing animals to protect human health.
Preface
The question of whether MAP can infect and cause disease in humans is one or today’s most hotly debated and controversial areas of MAP scientific inquiry. Since new scientific publications dealing directly or indirectly with this topic appear frequently, it is challenging to keep this part of the johnes.org website up to date. We are addressing this by formulating fairly short answers to the more common questions and linking to publications and websites that can lead interested readers to more detailed information and sources that are regularly updated. The long list of references (most linked to original research articles) appears at the bottom of this page and is updated frequently.
Common Questions:
- How can humans be exposed to MAP?
- Does MAP infect humans?
- Does MAP infection cause Crohn’s disease?
- What are the arguments for MAP as a cause of Crohn’s disease?
- What are the arguments against MAP as a cause of Crohn’s disease?
- Is there a genetic component to acquiring Crohn’s disease?
- Is genetics relevant to the theory that MAP contributes to Crohn’s disease?
- Has MAP been described as a factor in human ailments other than Crohn’s disease?
1. How can humans be exposed to MAP?
Before listing potential routes of human exposure, it is useful to recap MAP’s life history. The main reservoir for MAP in nature is infected animals. MAP is considered an obligate pathogen, meaning that it replicates in infected animals. Among animals, MAP is found primarily in ruminants such as cattle, sheep, and goat. (Non-ruminant animals such as omnivores and carnivores are infrequently infected with MAP and the infection rarely progresses to disease.) Ruminant infections begin when MAP is swallowed. MAP then invades the intestinal wall, invades white blood cells called macrophages and starts multiplying. As the infection slowly progresses (often many years pass before an animal appears sick), MAP spreads from the intestine to multiple other tissues and organs in the animal via the blood stream. The liver, spleen and lymph nodes filter MAP from the blood stream. Inside the animal the highest number of MAP can generally be found at the initial site of infection, the terminal small intestine (ileum) and in lymph nodes.
An infected ruminant excretes MAP in its manure . The organism may also appear in the infected ruminant’s colostrum and milk, but in far fewer numbers. Typical farm manure management practices may result in contamination of soil and water with MAP.
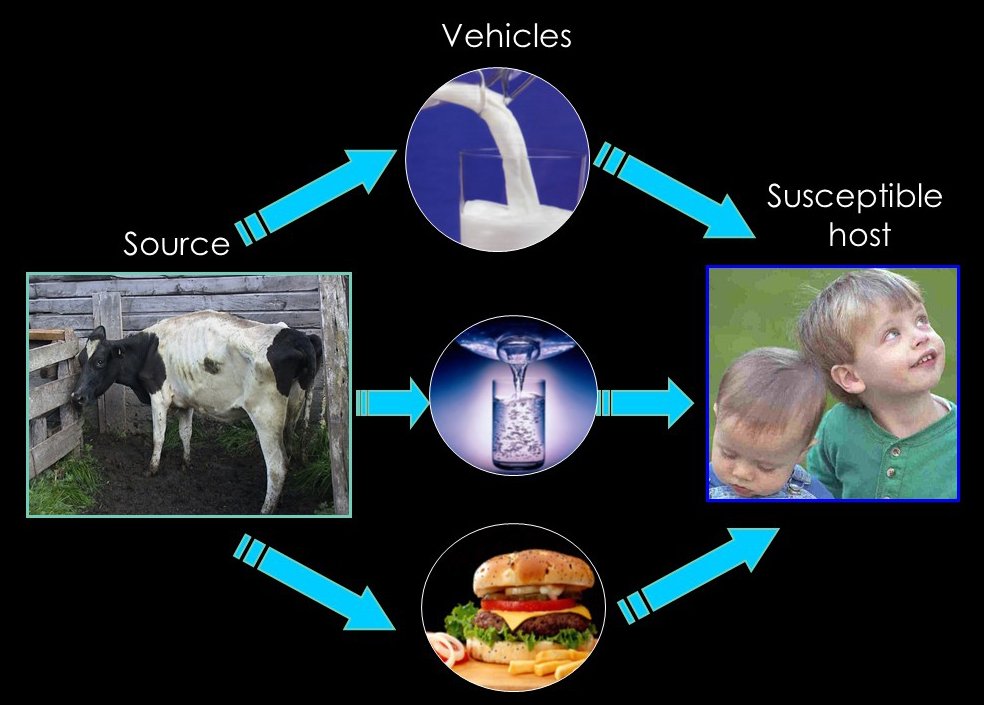
Given this natural history of MAP and food manufacturing practices, the following can be potentially MAP-contaminated and result in human exposure (ranked in decreasing order of probable importance):
- Raw milk from MAP-infected dairy herds.
- Ground beef originating from MAP-infected dairy cattle sold for slaughter.
- Process meat products from MAP-infected cattle, goats and sheep.
- HTST pasteurized milk: MAP is know to survive at low numbers in pasteurized milk.
- Domestic water originating from surface sources vulnerable to runoff from MAP-infected farms.
- Contact with small ruminants in petting zoos without adequate hand sanitizing facilities.
- Cuts of beef originating from MAP-infected beef cattle.
Note: It is unknown how many exposures are needed and how many MAP may be required to initiate infection in a human, or even whether all humans are susceptible to infection. Therefore, it is difficult to predict the importance of if these potential routes of exposure. Also, the age of exposure is likely to be as important in humans as it is in all other animals.
2. Does MAP infect humans?
Yes. Two studies, one authored by Abubakar et al. and the other by Feller et al., have assembled publications on studies searching for MAP, or immune responses to MAP, in humans -to establish whether MAP ever infects humans (see publications listed below). Both have concluded that MAP has infected humans although whether these infections subsequently were the direct cause of disease is an open question. In the cases when MAP could be isolated from humans, the genetic fingerprints of the organism were the same as those cultured from animals.
Note: Finding evidence of MAP infection does not necessarily indicate that MAP can cause disease in humans. The causation question remains unresolved.
3. Does MAP infection cause Crohn’s disease?
In the majority of published studies, evidence of MAP is found more often in people with Crohn’s disease than in other human populations. It is unknown if Crohn’s disease patients developed their disease first and then acquired the MAP infection or whether MAP actually contributed to their disease. Experimental infection trials in humans are unethical so there are no direct ways of proving that MAP causes Crohn’s disease. Evidence will always be indirect or circumstantial. However, the ability to cure Crohn’s disease using anti-MAP antibiotics, if proven, will be highly significant evidence supporting MAP as the cause.
4. What are the arguments for MAP as a cause of Crohn’s disease?
Borrowing from the publication by Sartor in 2005 (see reference list below), the following observations based on several studies support the hypothesis that MAP is one of the causes of Crohn’s disease:
- Crohn’s disease in humans is similar to Johne’s disease in animals both clinically and pathologically.
- MAP has been documented to infect nonhuman primates.
- Human exposure to MAP is plausible by multiple routes (see #1 above).
- Evidence of MAP is detected in tissues of Crohn’s disease patients more often than controls (see #2 above).
- Evidence MAP is detected in the blood of patients with Crohn’s disease more often than controls.
- Crohn’s disease has become a global disease with accelerating incidence in newly industrialized countries whose societies have become more westernized. This pattern is that an infectious disease, not a genetically-mediated autoimmune disorder.
- The genotypes of MAP found in animals are the same as those found in humans.
- Crohn’s disease patients have antibody to MAP in their blood more often than controls.
- Some Crohn’s patients have clinically improved when treated with antibiotics effective against a broad range of bacteria, including MAP. Based on a press release from RedHill Biopharma, a placebo-controlled, double-blind trial of a three antibiotic combination has shown to cause high remission rates in Crohn’s patients. A refereed scientific publication describing the trial is expected in 2021. This website has a personal story of a veterinarian cured of Crohn’s disease. The Human Para Foundation also has patient success stories.
5. What are the arguments against MAP as a cause of Crohn’s disease?
Borrowing again from the publication by Sartor in 2005 (see reference list below), the following observations support the hypothesis that MAP is NOT one of the causes of Crohn’s disease:
- There are some differences in histopathology between Johne’s disease in animals and Crohn’s disease in humans. (It should be noted that the pathology of zoonotic infections is seldom the same in animals and humans).
- There is no apparent risk of developing Crohn’s disease for people regularly in contact with ruminants.
- There is considerable variability in MAP detection from humans among laboratories (i.e. one lab reports that MAP was detected, other labs using the same samples report that MAP was not found).
- MAP is not seen in tissues of Crohn’s patients using conventional stains for mycobacteria. (It should be noted that when cull dairy cows were examined by histopathology “Acid-fast organisms were identified in 7 of 78 (8.97%) and 6 of 106 (5.61%) culture-positive ileum and lymph node samples, respectively.” (Martinson, 2008).
- Crohn’s patients do not get worse when immunosuppressed by drugs or due to HIV infection. [Immunosuppression generally causes microbial infections to worsen. In fact, immunosuppressed patients are more susceptible to mycobacterial infections.]
- Some Crohn’s patients have not clinically improved when treated with antibiotics effective against a broad range of bacteria, including MAP.
6. Is there a genetic component to acquiring Crohn’s disease?
Yes. Multiple genes have been associated with the development of Crohn’s disease. The two most significant are called NOD2 and ATG16L1. It is unknown exactly how or why these genes are linked to Crohn’s disease. It is known, however, that these genes regulate the human immune response to intracellular bacterial infections with organisms such as mycobacteria.
7. Is genetics relevant to the theory that MAP contributes to Crohn’s disease?
Yes. The same genes linked to Crohn’s disease susceptibility are also linked to mycobacterial and other intracellular bacterial infection susceptibility.
8. Has MAP been described as a factor in human ailments other than Crohn’s disease?
Yes. MAP was reported as the cause of lymphadenitis in a child and was detected in a patient with AIDS. In addition, MAP or an immune response to MAP has been found in cases of sarcoidosis, Blau syndrome (eye condition), Type I Diabetes Mellitus, and multiple sclerosis. The Human Para Foundation maintains a website devoted exclusively to human diseases linked with MAP.
Governmental Reports for Download
 A review of evidence for a link between exposure to Mycobacterium paratuberculosis and Crohn’s disease in humans. A report for the U.K. Foods Standards Agency.
A review of evidence for a link between exposure to Mycobacterium paratuberculosis and Crohn’s disease in humans. A report for the U.K. Foods Standards Agency.
Dr. Eileen Rubery, University of Cambridge, June, 2001
This 55 page document is a report by a scientist independently contracted to evaluate the M. paratuberculosis – Crohn’s disease link. The report is current, comprehensive, concise and lists 242 references. For those interested in this subject it is must reading.
Click here to download this 70 page 1 MB pdf document.
 Possible links between Crohn’s disease and paratuberculosis
Possible links between Crohn’s disease and paratuberculosis
The European Commission Directorate, March 21, 2000
This link provides access to The European Commission Directorate-General Health & Consumer Protection, Report of the Scientific Committee on Animal Health and Animal Welfare adopted 21 March 2000. This 76 page report is the product of a nine member panel of European experts. It provides an excellent review of the literature listing 372 references in its bibliography.
Click here to download this 76 page 1 MB pdf file.
Websites related to MAP in humans

Prof. Hermon-Taylor, together with Dr Tim Bull and other members of the team at St George’s University of London and scientists at the Jenner Institute University of Oxford, developed a modern DNA vaccine against MAP. The Crohn’s MAP Vaccine team supports this ground-breaking work led by Dr Amy Hermon-Taylor (Prof. Hermon-Taylor’s daughter) and a large group of volunteers all of whom are directly affected by Crohn’s disease.
Lay publications
The Crohn’s Connection by Lisa Chamberlain. Cleveland Free Times, June 16-22, 1999. Also available through Project Censored: Bacterium in cow’s milk may cause Crohn’s disease.
Paratuberculosis And Crohn’s Disease: Got Milk? by Michael Greger, MD, Updated January 2001.
Relevant Research Publications
Abubakar, I., Myhill, D., Aliyu, S.H., et al. 2008. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm. Bowel Dis. 14:401-410.
Acharya, K.R., Plain, K.M., Whittington, R.J. et al. 2020. Australian veterinarians’ perceptions regarding the zoonotic potential of Mycobacterium avium subspecies paratuberculosis. Veterinary Sciences 7, 33; doi:10.3390/vetsci7010033
Agrawal, G., Aitken, J., Hamblin, H. et al. 2020. Putting Crohn’s on the MAP: Five common questions on the contribution of Mycobacterium avium subspecies paratuberculosis to the pathophysiology of Crohn’s Disease. Digestive Diseases and Sciences https://doi.org/10.1007/s10620-020-06653-0
Agrawal, G., Clancy, A., Huynh, R., et al. 2020. Profound remission in Crohn’s disease no further treatment for 3-23 years: a case series. Gut Pathogens12:16.
Agrawal, G., Clancy, A., Sharma, R., et al. 2020. Targeted combination antibiotic therapy induces remission in treatment-naive Crohn’s disease: A case series. Microorganisms. 8, 371; doi:10.3390/microorganisms8030371.
Alonso-Hearn, M., Molina, E., Geijo, M., et al. 2009. Isolation of Mycobacterium avium subsp. paratuberculosis from muscle tissue of naturally infected cattle. Foodborne Pathog. Dis. 6:513-518.
Banche, G., Allizond, V., Sostegni, R., et al. 2015. Application of multiple laboratory tests for Mycobacterium avium ssp. paratuberculosis detection in Crohn’s disease patients specimens. New Microbiologica 38:357-367.
Behr, M.A. & Kapur, V. 2008. The evidence for Mycobacterium paratuberculosis in Crohn’s disease. Curr. Opinion Gastroenterol. 24:17-21.
Behr, M. A. & Hanley, J. Antimycobacterial therapy for Crohn’s disease: a reanalysis. 2008. Lancet Infect. Dis. 8:344. [this editorial refers to the Selby study cited below]
Behr, M.A. 2010. The path to Crohn’s disease: Is mucosal pathology a secondary event? Inflamm. Bowel Dis. 16: 896-902.
Bharathy S., Gunaseelan L., Porteen K. 2017. Exploring the potential hazard of Mycobacterium avium subspecies paratuberculosis as a cause for Crohn’s disease, Veterinary World, 10(4): 457-460.
Bull, T.J., McMinn, E.J., Sidi-Boumedine, K., et al. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J. Clin. Microbiol. 41:2915-2923.
Chamberlin, W., Ghobrial, G., Chehtane, M., et al. 2007. Successful treatment of a Crohn’s disease patient infected with bacteremic Mycobacterium paratuberculosis. Am. J. Gastroenterol. 102:689-691.
Davis, W.C. 2015. On deaf ears, Mycobacterium avium paratuberculosis pathogenesis in Crohn’s and other diseases. World J. Gastroenterol. 21(48): -0000. DOI: 10.3748/wjg.v21.i48.
Dow, C.T., & Sechi, L.A.. 2019. [Hypothesis] Cows Get Crohn’s Disease and They’re Giving Us Diabetes. Microorganisms 7(10):466 OPEN ACCESS
Eltholth, M.M., Marsh, V.R., Van Winden, S., et al. 2009. Contamination of food products with Mycobacterium avium paratuberculosis: a systematic review. J. Appl. Microbiol. 107:1061-1071.
Estevinho, M.M., Cabeda, J., Santiago, M., et al. 2023. Viable Mycobacterium avium subsp. paratuberculosis colonizes peripheral blood of inflammatory bowel disease patients. Microorganisms 2023, 11(6), 1520; https://doi.org/10.3390/microorganisms11061520
Feller, M., Huwiler, K., Stephan, R., et al. 2007. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect. Dis. 7:607-613.
Ghadiali, A.F., Strother, M., Naser, S.A. et al. 2004. Mycobacterium avium subsp. paratuberculosis strains isolated from Crohn’s Disease patients and animal species exhibit similar polymorphic locus patterns. J. Clin. Microbiol. 42:5345-5348.
Graham, D.Y., Naser, S.A., Borody, T. et al. 2024. Randomized, double-blind, placebo-controlled study of anti-mycobacterial therapy (RHB-104) in active Crohn’s disease. Antibiotics 13:694.
Greenstein, R.J. 2003. Is Crohn’s disease caused by a Mycobacterium? Comparisons with leprosy, tuberculosis, and Johne’s disease. Lancet Infect. Dis. 3:507-514.
Gupta, S., Chaubey, K.K., Agarwal, P., et al. Therapeutic management of Mycobacterium avium subspecies paratuberculosis infection with complete resolution of symptoms and disease in a patient with advanced inflammatory bowel syndrome. Molecular Biology Reports. https://doi.org/10.1007/s11033-021-06615-3.
Hermon-Taylor, J., Barnes, N., Clarke, C. Finlayson, C. 1998. Mycobacterium paratuberculosis cervical lymphadenitis, followed five years later by terminal ileitis similar to Crohn’s disease. British Medical Journal. 316:449-453 (February 7, 1998).
Hermon-Taylor, J., Bull, T.J., Sheridan, J.M. et al. 2000. Causation of Crohn’s disease by Mycobacterium avium subspecies paratuberculosis. Can. J. Gastroenterol. 14(6):521-539. [Mini-review with 369 references].
Hermon-Taylor, J. & Bull, T. 2002. Crohn’s disease caused by Mycobacterium avium subspecies paratuberculosis: A public health tragedy whose resolution is long overdue. J. Med. Microbiol. 51:3-6. [Editorial]
Honap, S., Johnston, E., Agrawal, G. et al. 2020. Anti-Mycobacterium paratuberculosis (MAP) therapy for Crohn’s disease: an overview and update. Frontline Gastroenterology 2020;0:1–7. doi:10.1136/flgastro-2020-101471.
Hruska, K., & Pavlik, I. 2014. Crohn’s disease and related inflammatory diseases: from many single hypotheses to one “superhypothesis”. Veterinarni Medicina 59(12):583-630. [REVIEW with 72 references]
Kirkwood, C.D., Wagner, J., Boniface, et al. 2009. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm. Bowel Dis. 15: 1643-1655.
Kuenstner, J.T., Kali, M., Welch, C. 2019. Whole exome sequencing of patients who resolved Crohn’s disease and complex regional pain syndrome following treatment for paratuberculosis. Gut Pathogens volume 11, Article number: 34. OPEN ACCESS
Kuenstner, J.T., Potula, R., Bull, T., et al. 2020. Presence of infection by Mycobacterium avium subsp. paratuberculosis in the blood of patients with Crohn’s disease and control subjects shown by multiple laboratory culture and antibody methods. Microorganisms 2020 8,2054; doi:10.3390/microorganisms8122054
Lidar, M., Langevitz, P., & Shoenfeld, Y. 2009. The role of infection in inflammatory bowel disease: initiation, exacerbation and protection. Isr. Med. Assoc. J. 11:558-563.
Mendoza, J.L., San-Pedro, A., Culebras, E., et al. 2010. High prevalence of viable Mycobacterium avium subspecies paratuberculosis in Crohn’s disease. World J. Gastroenterol. 16(36): 458-4563.
Mutharia, L.M., Klassen, M.D., Fairles, et al. 2010. Mycobacterium avium subsp. paratuberculosis in muscle, lymphatic and organ tissues from cows with advanced Johne’s disease. Int. J. Food Microbiol. 136:340-344.
Nacy, C. & Buckley, M. 2008. Mycobacterium avium paratuberculosis: Infrequent human pathogen or public health threat? American Society for Microbiology, Washington DC, USA.
Naser, S.A., Collins, M.T., Crawford, J.T., et al. 2009. Culture of Mycobacterium avium subspecies paratuberculosis (MAP) from the blood of patients with Crohn’s disease: A follow-up blind multi-center investigation. The Open Inflamm. J. 2: 22-23.
Naser, S.A., Ghobrial, G., Romero, C., et al. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 364:1039-1044.
Naser, S.A., Sagramsingh, S.R., Naser, A.S., et al. 2014. Mycobacterium avium subspecies paratuberculosis causes Crohn’s disease in some inflammatory bowel disease patients. World J. Gastroenterol. 20:7403-7415.
Pal, M., Alema, J., Rahman, T. 2015. Mycobacterium avium subspecies partuberculosis: An emerging bacterial disease of global public health significance. Microbes and Health 4(1):4-13. [Review with 68 references]
Romero, C., Hamdi, A., Valentine, J.F. et al. 2005. Evaluation of surgical tissue from patients with Crohn’s Disease for the presence of Mycobacterium avium subspecies paratuberculosis DNA by in situ hybridization and nested polymerase chain reaction. Inflamm. Bowel Dis. 11:116-125.
Ryan, P., Bennett, M.W., Aarons, S. et al. 2002. PCR detection of Mycobacterium paratuberculosis in Crohn’s disease granulomas isolated by laser capture microdissection. Gut 2002;51:665–670.
Sartor, R.B. 2005. Does Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease? Gut 54:896-898.
Savarino, E.L. Bertani, L. Ceccarelli, et al. 2019. Antimicrobial treatment with the fixed-dose antibiotic combination RHB-104 for Mycobacterium avium subspecies paratuberculosis in Crohn’s disease: pharmacological and clinical implications. Expert Opinion on Biological Therapy, 19:2, 79-88, DOI: 10.1080/14712598.2019.1561852.
Scanu, A.M., Bull, T.J., Cannas, S. et al. 2007. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn’s disease and Johne’s disease: Common neural and immune pathogenicities. J. CLin. Microbiol. 45:3883-3890.
Schurr, E. & Gros, P. 2009. A common genetic fingerprint in leprosy and Crohn’s disease? New Engl. J. Med., December 16, 2009.
Sechi, L.A., Dow, C.T.. 2015. Mycobacterium avium ss. paratuberculosis zoonosis – The hundred year war – beyond Crohn’s disease. Front. Immunol. Minireview – article 96, 8 pages with 117 references doi: 10.3389/fimmu.2015.00096.
Sechi, L.A., Gazouli, M., Ikonomopoulos, J., et al. 2005. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn’s disease, and Sardinians: A way ahead. J. Clin. Microbiol. 43: 5275-5277.
Selby, W., Pavli, P., Crotty, et al. 2007. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterol. 132:2313-2319.
Sibartie, S., Scully, P., Keohane, J., et al. 2010. Mycobacterium avium subsp. paratuberculosis (MAP) as a modifying factor in Crohn’s Disease. Inflamm. Bowel Dis. 16:296-304.
Vogt, L.M. 2016. Crohn’s Disease: A case for MAP targeted therapy. [Opinion] EC Microbiology 1.S1 (2016): S1-S4.
Waddell, L.A., A Rajic, Stark, K.D.C., McEwen, S.A. 2016. The potential public health impact of Mycobacterium avium ssp. paratuberculosis: Global opinion survey of topic specialists. Zoonoses and Public Health, 63:212–222.
Zarei-Kordshouli, F., Geramizadeh, B.,Khodakaram-Tafti, A. 2019. Prevalence of Mycobacterium avium subspecies paratuberculosis IS 900 DNA in biopsy tissues from patients with Crohn’s disease: histopathological and molecular comparison with Johne’s disease in Fars province of Iran. BMC Infect Dis 19, 23 doi:10.1186/s12879-018-3619-2. OPEN ACCESS
Zhang, F.-R., Huang, W., Chen, S.-M. et al. 2009. Genomewide association study of leprosy. New Engl. J. Med. 361:2609-2618.
Zhang, P., Minardi, L.M. Kuenstner, J.T., et al. 2020. Serological testing for mycobacterial heat shock protein Hsp65 antibody in health and diseases. Microorganisms. doi:10.3390/microorganisms8010047.