Dr. David Graham from Baylor College of Medicine together with 16 international colleagues published the results of a large trial to treat Crohn’s disease with anti-MAP antibiotics. This study met the highest standards for a clinical trial, namely it was a multi-center randomized, double-blind, placebo-controlled study. This clinical trial was conducted at 92 sites in the United States, Canada, Bulgaria, the Czech Republic, Australia, New Zealand, Israel, Poland, Serbia, and Slovakia. The article was published in the journal Antibiotics and is Open Access (free for everyone).
ABSTRACT
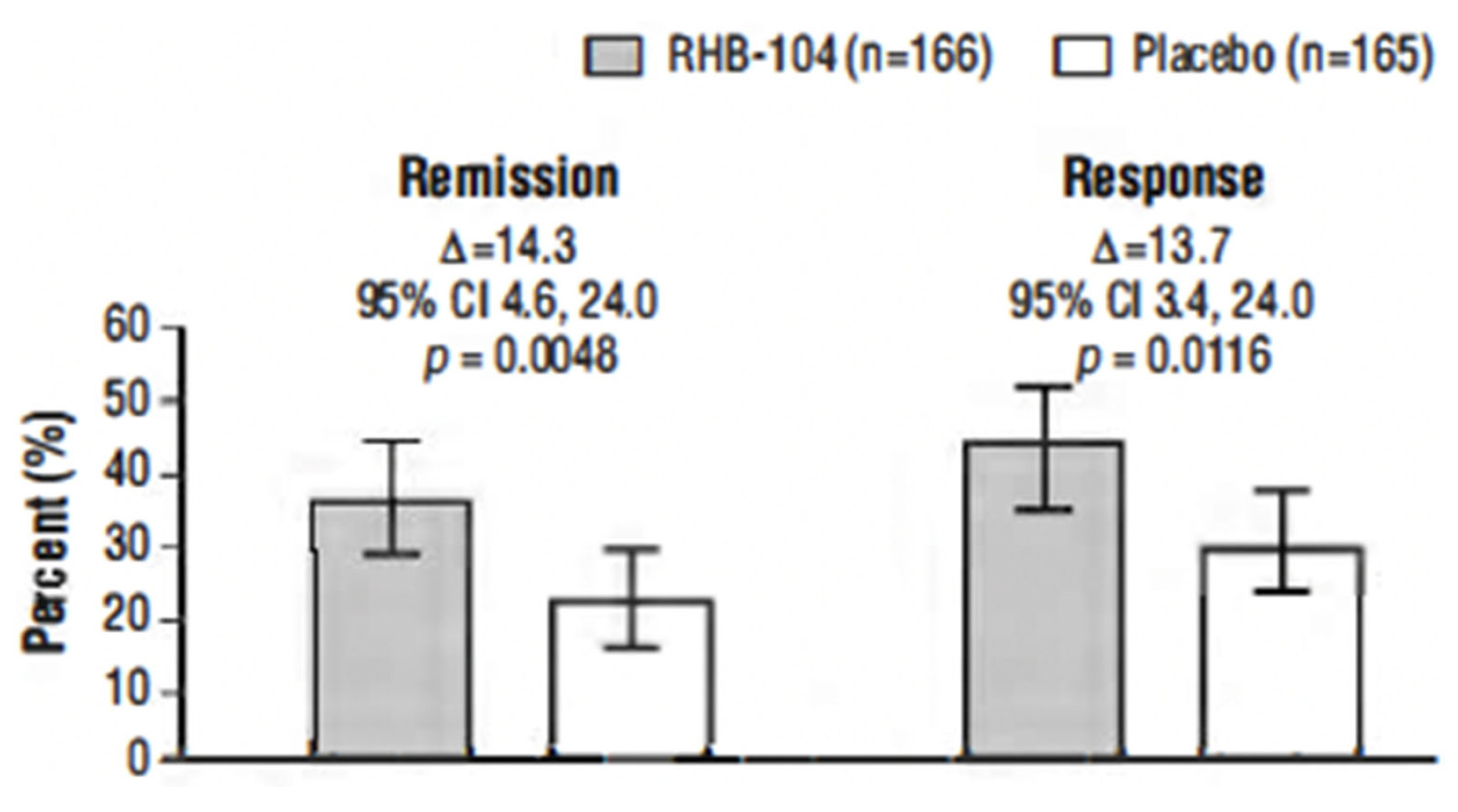
This study, conducted between 4 October 2013, and 30 November 2018, tested the hypothesis that triple antimicrobial therapy, targeting Mycobacterium avium subspecies paratuberculosis (MAP), long considered a putative cause, would favorably affect Crohn’s disease. A double-blind multicenter study of adults with active Crohn’s disease, (i.e., Crohn’s Disease Activity Index [CDAI] 220–450 plus C-reactive protein ≥ 1.0 mg/dL, fecal calprotectin (FCP) >162.9 μg/g stool, or recent endoscopic or radiographic confirmation of active disease) receiving concomitant standard-of-care Crohn’s disease treatment (Clinicaltrials.gov: NCT01951326) were stratified by anti-tumor necrosis factor use and randomized (1:1) to anti-MAP RHB-104 (clarithromycin 95 mg, rifabutin 45 mg, and clofazimine 10 mg per capsule) (n = 166), resulting in clarithromycin 950 mg/day, rifabutin 450 mg/day, and clofazimine 100 mg/day, or placebo (n = 165) for up to 52 weeks. A greater proportion of RHB-104 versus placebo-treated patients met the primary endpoint—remission (i.e., CDAI < 150)—at week 26 (36.7% [61/166] vs. 22.4% [37/165], respectively; 95% CI for difference: 4.6, 24.0, p = 0.0048; chi-square test). Clinical response (reduction of CDAI by ≥100 points from baseline) at week 26 (first secondary endpoint) was also higher among the patients treated with RHB-104 (73/166 [44.0%]) compared with placebo (50/165 [30.3%]; 95% CI for difference: 3.4, 24.0, p = 0.0116), and it remained higher at week 52 among the patients treated with RHB-104 (59/166 [35.5%] vs. (35/165 [21.2%] for placebo; 95% CI for difference: 4.7, 23.9, p = 0.0042). A statistically significantly greater decline in FCP (another prospective efficacy endpoint) was also observed in RHB-104-treated patients, compared with placebo, at weeks 12, 26, and 52. The rates of serious adverse events were similar between groups (RHB-104: 18.7%; placebo: 18.8%). No patient died during the study. Antimicrobial therapy directed against MAP resulted in significantly greater improvement in clinical and laboratory (FCP) measures of active Crohn’s disease.
COMMENTS
There have been multiple scientific reports on curing individual cases of Crohn’s disease using anti-MAP antibiotics. The article in the journal Antibiotics is the largest clinical trial testing a treatment for Crohn’s disease directed at a cause of Crohn’s disease, namely MAP, rather than at the symptoms of the disease, e.g. immunosuppressive drugs. It significantly adds to the body of evidence that MAP is a zoonotic bacterial pathogen causing chronic intestinal inflammation in a diverse array of animal species.
For a true story of a veterinarian permanently cured of his Crohn’s disease, and links to similar case reports, visit this page of the website: https://johnes.org/other-animals/non-ruminants/
For more on the zoonotic potential of MAP, and links to scientific articles on the subject, visit this page of the website: https://johnes.org/zoonotic-potential/.
For more on MAP in food and water visit this page: https://johnes.org/is-map-in-food-and-water/.
For a comprehensive 90-minute lecture on scientific evidence that MAP is a cause of Crohn’s disease see this page: https://johnes.org/presentations-and-mini-lectures/