 Management decisions to protect calves from infection such as separation of infected cows at calving and discard of calves, milk and colostrum from MAP positive cows, or pasteurization of their milk, are uncommon in seasonal, pastoral New Zealand (NZ) dairy farming. Pasture management is greatly complicated by any increase in the number of groups of grazing cows. The NZ Animal Compounds and Veterinary Medicines act (1987) prohibits the sale of milk for human consumption when that milk is contaminated with drug residues. Consequently, calves are commonly fed on milk from sick cows, those undergoing antimicrobial treatment or excluded from the main herd for other reasons.
Management decisions to protect calves from infection such as separation of infected cows at calving and discard of calves, milk and colostrum from MAP positive cows, or pasteurization of their milk, are uncommon in seasonal, pastoral New Zealand (NZ) dairy farming. Pasture management is greatly complicated by any increase in the number of groups of grazing cows. The NZ Animal Compounds and Veterinary Medicines act (1987) prohibits the sale of milk for human consumption when that milk is contaminated with drug residues. Consequently, calves are commonly fed on milk from sick cows, those undergoing antimicrobial treatment or excluded from the main herd for other reasons.
Therefore, in NZ, there has been relatively little engagement from dairy farmers in the control of JD unless they have experienced a high clinical prevalence and there is evidence of increasing prevalence of JD especially in the South Island of the country.
Andrew Bates et al., Vetlife Centre for Dairy Excellence, Geraldine, NZ reported in BMC Veterinary Research (Open Access) the results of a single herd study where a high prevalence of clinical JD and MAP infection was reduced over a 4 year period using an annual test and cull approach. The strategy was based on a herd testing protocol using an initial herd screening using serological ELISA coupled with a quantitative fecal PCR (fPCR) test to confirm the status of ELISA-positive animals.
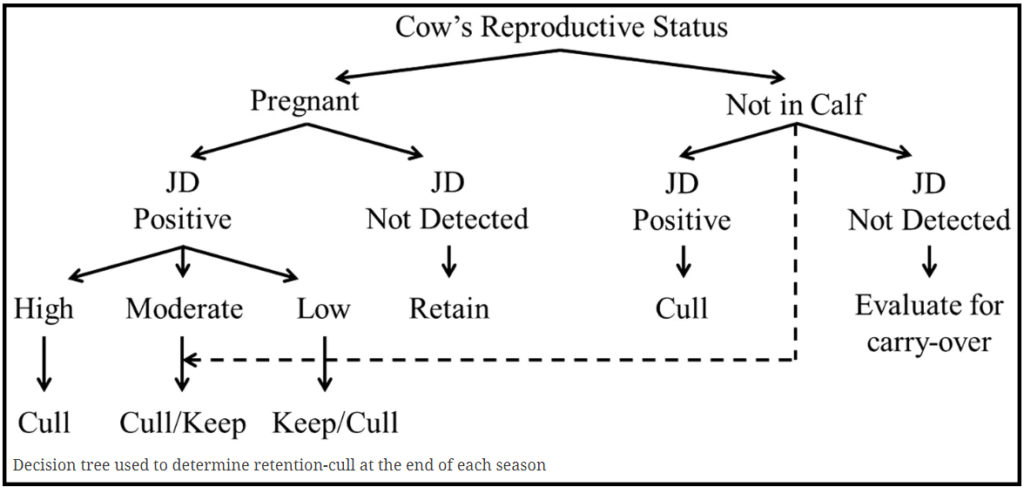
Over the 4 year period a total of 4,358 blood samples were submitted from 2,211 cows and of these 683 were submitted for fPCR. Culling decisions followed a decision tree approach shown below. To aid the removal of animals shedding large numbers of MAP, a priority was made to remove all animals with a high fPCR status, followed by those that were had high ELISA results.
For all age groups considered the apparent seroprevalence of cows testing positive decreased from 26% in 2013–2014, to 2.3% in 2016–2017. The reported proportion of calved cows culled annually from suspected clinical Johne’s disease fell from 5% in the year preceding the control program to 0.4% in the final year of the study.
On this farm, reduction in the prevalence of infection was achieved by reducing the infectious pressure through targeted culling of heavily shedding animals together with limited measures to protect calves at pasture from exposure to Mycobacterium avium subsp. paratuberculosis (MAP).
This study demonstrates that – with a combination of pre-calving diagnostic testing to identify and remove animals that are the major source of infectious spread, coupled with simple management changes to physically separate replacement calves from MAP infected adult cattle – effective reduction in the prevalence of JD is possible for NZ dairy farmers.
Comment: This important study validates that removing the most infectious animals from a herd significantly decreases the prevalence of the infection. However, it ignores the economic utility of the program, i.e. the cost-benefit of this approach. If the ELISA was valued at US$5.00 and the fPCR at US$30 (typical costs in U.S. labs), then the cost of this control program, in laboratory testing costs alone, was $42,280. Most herd owners would question the return on investment without receiving some compensation from the processor buying his/her milk or from a governmental agency concerned with food safety.