 F. Biemans from the Centre for Veterinary Epidemiology and Risk Analysis, UCD School of Veterinary Medicine, University College Dublin, Ireland together with colleagues from INRAE, Oniris, BIOEPAR, Nantes, France and Teagasc, Oak Park, Carlow, Ireland, published a study titled: Modelling transmission and control of Mycobacterium avium subspecies paratuberculosis within Irish dairy herds with compact spring calving. Their article appears in the January 2021 issue of Preventive Veterinary Medicine.
F. Biemans from the Centre for Veterinary Epidemiology and Risk Analysis, UCD School of Veterinary Medicine, University College Dublin, Ireland together with colleagues from INRAE, Oniris, BIOEPAR, Nantes, France and Teagasc, Oak Park, Carlow, Ireland, published a study titled: Modelling transmission and control of Mycobacterium avium subspecies paratuberculosis within Irish dairy herds with compact spring calving. Their article appears in the January 2021 issue of Preventive Veterinary Medicine.
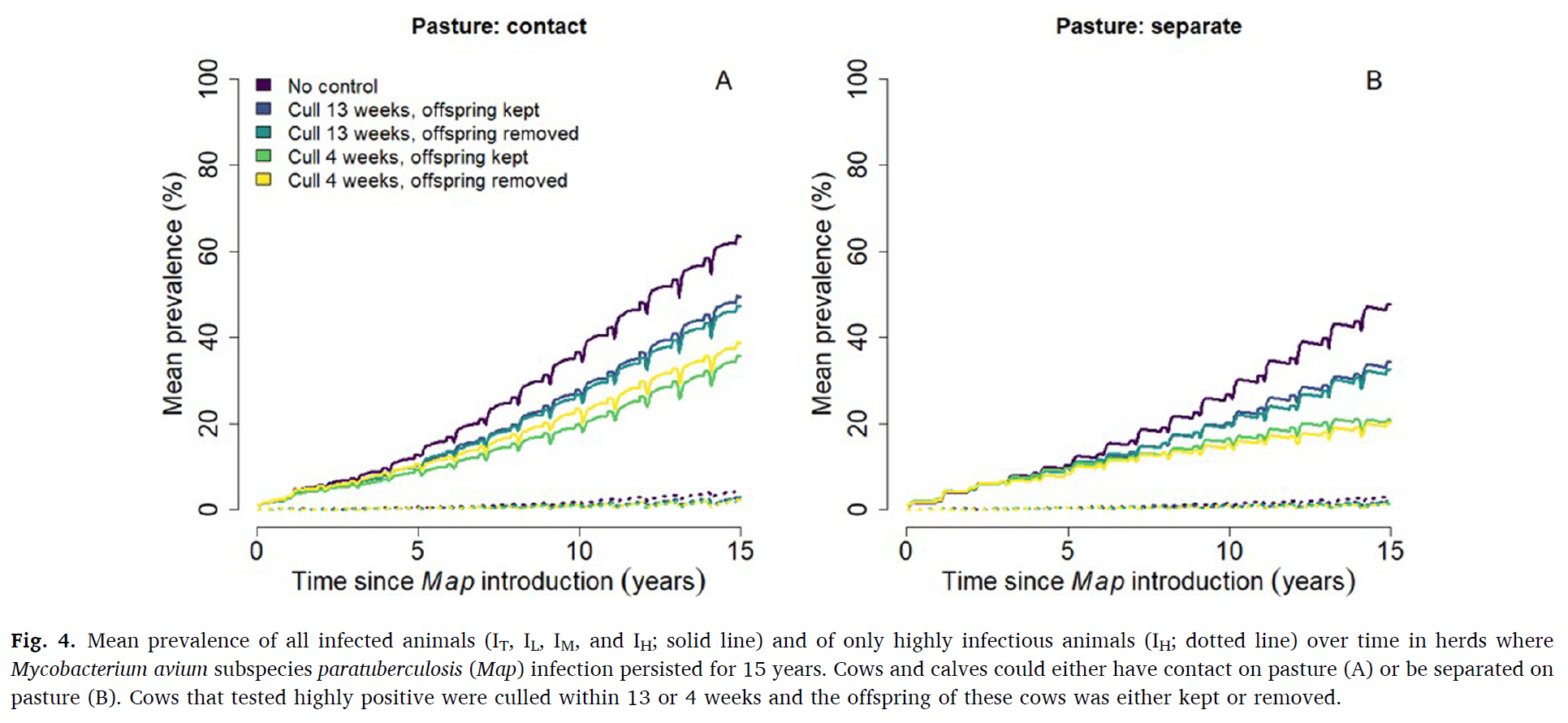
This graphic from their publication shows the spread of Johne’s disease in grazing dairy herds with and without appropriate control measures. Annual testing by serum ELISA, prompt culling of the high (strong) ELISA-positive cows and separation of the calf from the cow soon after birth were critically important control measures.
Abstract
Paratuberculosis is a chronic bacterial infection of the intestine in cattle caused by Mycobacterium avium subspecies paratuberculosis (Map). To better understand Map transmission in Irish dairy herds, we adapted the French stochastic individual-based epidemiological simulation model to account for seasonal herd demographics. We investigated the probability of Map persistence over time, the within-herd prevalence over time, and the relative importance of transmission pathways, and assessed the relative effectiveness of test-and-cull control strategies.
We investigated the impact on model outputs of calf separation from cows (calves grazed on pasture adjacent to cows vs. were completely separated from cows) and test-and-cull. Test-and-cull scenarios consisted of highly test-positive cows culled within 13 or 4 weeks after detection, and calf born to highly test-positive cows kept vs removed. We simulated a typical Irish dairy herd with on average 82 lactating cows, 112 animals in total. Each scenario was iterated 1000 times to adjust variation caused by stochasticity. Map was introduced in the fully naive herd through the purchase of a moderately infectious primiparous cow. Infection was considered to persist when at least one infected animal remained in the herd or when Map was present in the environment.
The probability of Map persistence 15 years after introduction ranged between 32.2–42.7% when calves and cows had contact on pasture, and between 18.9–29.4% when calves and cows were separated on pasture. The most effective control strategy was to cull highly test-positive cows within four weeks of detection (absolute 10% lower persistence compared to scenarios without control). Removing the offspring of highly test-positive dams did not affect either Map persistence or within-herd prevalence of Map.
Mean prevalence 15 years after Map introduction was highest (63.5 %) when calves and cows had contact on pasture. Mean prevalence was 15 % lower (absolute decrease) when cows were culled within 13 weeks of a high test-positive result, and 28 % lower when culled within 4 weeks. Around calving, the infection rate was high, with calves being infected in utero or via the general indoor environment (most important transmission routes). For the remainder of the year, the incidence rate was relatively low with most calves being infected on pasture when in contact with cows. Testing and culling was an effective control strategy when it was used prior to the calving period to minimize the number of highly infectious cows present when calves were born.
Comments
Animal husbandry systems heavily influence the options for Johne’s disease control measures. This excellent publication is focus on the type of pastoral or gazing type of dairy herd management prevalent in Ireland. It reinforces the importance of culling the cows with high-positive serum ELISA results and prompt separation of calves from cows after birth. Very interested readers should read the section on model assumptions (section 3.7 on page 8) to judge whether they model fits dairy herd management systems in other their country. In the book Empirical Model-Building and Response Surfaces by Box and Draper (1987) they state: “Essentially, all models are wrong, but some are useful.” I would rank this model as very useful.
Footnote
ELISAs measure the quantity of antibody in the clinical sample which can be either serum (from blood) or milk (for dairy cows). The ELISA reports numerical results called S/P or S/P% values and values above a certain cut-off are classified as positive. However, much more useful information, beyond positive or negative interpretations, can be had when you examine the magnitude of the ELISA result. Animals in the high range, typically with S/P values over 1.0 or S/P% values over 100 are consider “high-positive”, also called “strong-positive”. Multiple studies have shown that this is important information as cows with high-positive ELISA results are the ones most likely to be shedding the most MAP in their feces and milk and most likely to have infected their unborn fetus. Thus, these are the first cows among all of the ELISA-positive animals that should be culled.