T.C. Ekundayo and colleagues have published a systematic review and meta-analysis on MAP as a trigger for multiple sclerosis (MS). The article (in press) appears in the journal Multiple Sclerosis and Related Disorders. The abstract appears toward the end of this news post. For non-experts to fully understand this study and its implications first requires some background information. Links are provided for those wanting more subject matter depth.
Multiple Sclerosis (MS) – extracted from Wikipedia
MS is an autoimmune disease (the body attacks itself) and is the most common demyelinating disease. Myelin in the material that provides insulation around nerve fibers and when it is lost (demyelination) the nerves cannot effectively transmit signals. The clinical signs of MS vary depending on which nerves are affected. Sensations such as tingling, or numbness and muscle weakness are common signs when peripheral nerves are affected. Vision and psychological problems also can occur when nerves in the brain are affected. Symptoms occur either as episodes of sudden worsening that last a few days to months (called relapses or flare-ups) followed by improvement (85% of cases) or as a gradual worsening over time without periods of recovery (10–15% of cases).
The cause of MS is unknown but believed to occur because of some combination of genetic and environmental factors such as infectious agents. MS is more common in people who live farther from the equator. Environmental factors may play a role during childhood. People who move to a different region of the world before the age of 15 acquire the new region’s risk to MS. This is worth noting since there is an age-dependent susceptibility of animals, and quite likely humans, to MAP infection.
MS is not considered a hereditary disease; however, a number of genetic variations have been shown to increase the risk. Some genes linked to MS have also been implicated in other autoimmune diseases such as Type 1 Diabetes (another disease linked to MAP).
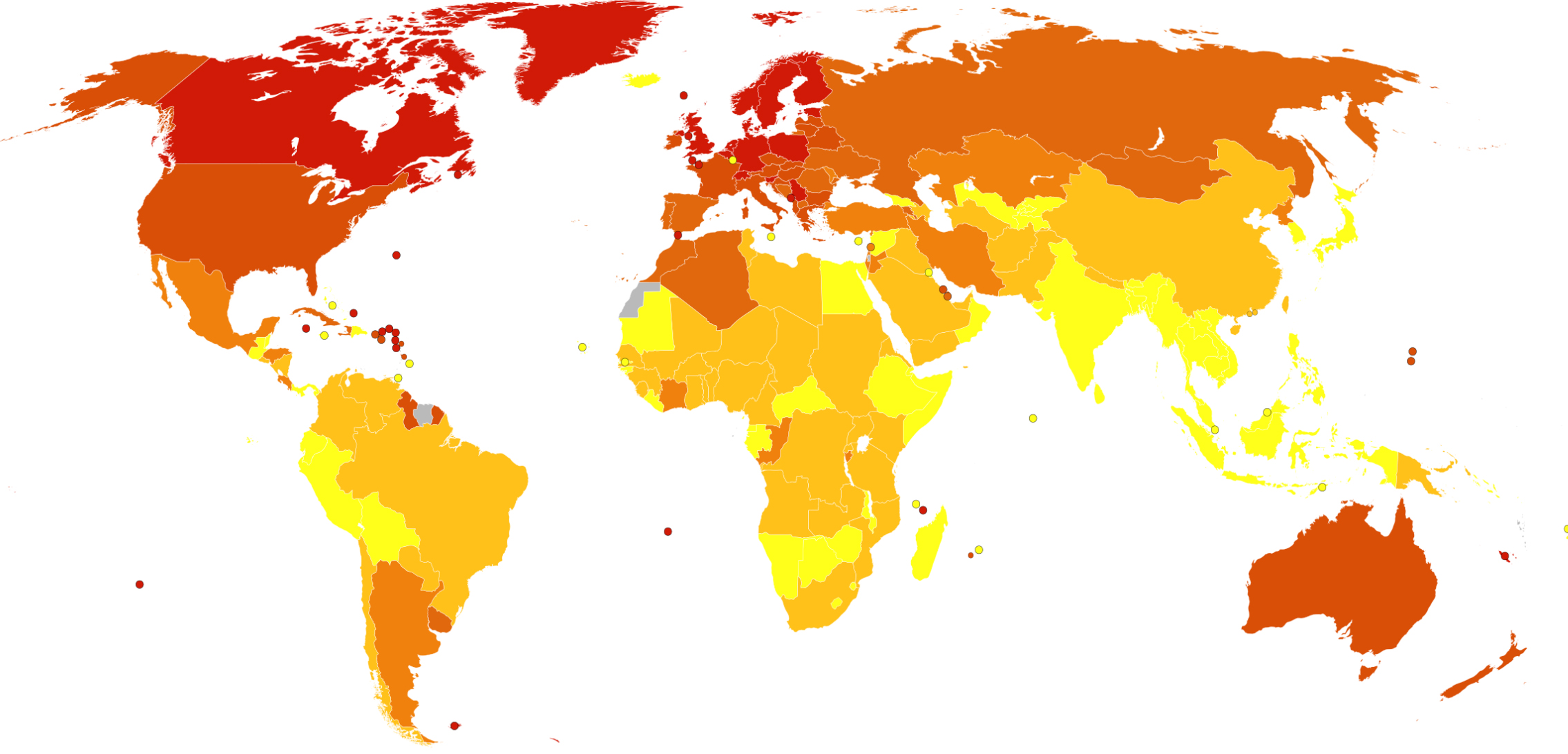
MS usually appears in adults in their late 20s or early 30s. As of 2010, the number of people with MS was 2–2.5 million (approximately 30 per 100,000) globally, with rates varying widely in different regions. The map below from Wikipedia shows death rates from MS per million people by country. The incidence of MS appears to be increasing.
Autoimmune diseases with bacterial triggers
The details of how and why microbes can trigger autoimmunity are complex and not fully agreed on. Molecular mimicry is one of the better understood mechanisms. In this situation, a microbe produces an antigen that shares structural similarities with antigens on specific host tissues. A chemical component of the pathogen mimics the structure of antigens on human tissues and the immune system fails to distinguish between the microbe and the host’s own tissues. When antibodies produced against this mimic microbial antigen bind to the host tissues, inflammation and disease can result. Rheumatic fever, which follows infection of people, most often children, with Group A Streptococcus bacteria (commonly known as “Strep throat”), is a well-known example. Rheumatic fever can be prevented by prompt treatment of Strep throat with appropriate antibiotics. Rheumatic fever is well-controlled in developed countries but remains a scourge elsewhere.
Food poisoning caused by Campylobacter jejuni (most often associated with under cooked poultry) is an interesting example because it also involves autoimmunity and the nervous system, as in MS. C. jejuni is one of the most common causes of food poisoning in Europe and in the United States. After a C. jenuni infection, a small proportion of people produce white blood cells (T-lymphocytes) and antibodies direct against parts of the C. jejuni cell wall that structurally resemble chemicals surrounding human nerves. When these antibodies mistakenly bind to the insulating material around nerve cells, inflammation occurs leading to muscle paralysis which is a condition called Guillain-Barré syndrome (GBS). The paralysis is typically reversible; nonetheless, about 20% of patients with GBS are left disabled, and around 5% die. Campylobacter infections can occur in all age groups. Studies show a peak incidence in children younger than 1 year and in people aged 15–29 years. Only about 1 in 2,000 people with a C. jejuni infection develop Guillain-Barré syndrome; essentially a combination of host genetics, level of immunity to C. jejuni infection from prior infections, C. jejuni biology, and bad luck. Because of the impact on human health, the poultry industry works hard to limit C. jejuni contamination of poultry products in the slaughterhouse (abattoir) and regulatory agencies, such as the USDA, monitor C. jejuni levels in retail poultry products.
MAP as a trigger for MS
Back to the topic of MAP and MS. T.C. Ekundayo and colleagues conducted a systematic review and meta-analysis on the hypothesis that MAP is a trigger for multiple sclerosis (MS). In lay terms, this means that they gathered all the available published scientific literature on the subject using specific scientific methodology and then used well-define statistical methods for judging the outcomes of those studies; essentially looking for scientific consensus. Such studies are daunting to read and understand for non-experts. However, the conclusions should be given considerable weight because they are the compilation of many studies done in different countries by diverse scientists. The authors concluded that there is a “strong association between MAP and MS, indicating that MAP is a significant environmental agent that may trigger MS.” The abstract of the publication is below and truly interested readers are encouraged to seek out the primary literature cited in the 42 references listed at the end of the publication.
ABSTRACT
Mycobacterium avium subsp. paratuberculosis (MAP) has been identified as one of the environmental infectious agents that causes multiple sclerosis (MS). The global prevalence of MS has been up surging over the years; however, efforts to divulge the role of MAP in MS have been limited. As a result, the present study aimed at assessing the odd ratios (ORs) associated MAP with the risk of MS. MAP-related MS data were obtained from 6 databases using the terms ‘multiple sclerosis’ or ‘MS’ and ‘paratuberculosis’ without regard for time or language restrictions following PRISMA standards. A total of 2,538 participants’ data from 12 studies presenting anti-MAP antibodies and MAP DNA from 4 studies were fitted in random-effects (RE) and fixed-effects (FE) meta-analytic models. Furthermore, the between-study heterogeneity was measured using I2-values with a significant limit set at an I² > 75%. Analytical rigour and publication bias was determined using leave-one-out-analytics, Egger’s tests, and p-curve analysis. In the FE and RE models, anti-MAP antibodies data significantly associated MS risk with MAP as 10.71 OR (95%-CI [7.78; 14.74], p-value < 0.0001) and 12.76 OR (95%-CI [8.13; 20.02], p-value < 0.0001) respectively, with an I2 value of 34.9% (95%-CI [0.0%; 67.2%]; p-value = 0.11). Similarly, the MAP DNA dataset in FE significantly present MS risk due to MAP as 5.53 OR (95%-CI [3.54; 8.66], p-value< 0.0001) while, RE showed 5.27 OR (95%-CI [3.22; 8.60], p = 0.0017), with an I2-value = 0.0% (95%- CI [0.0%; 84.7%]; p-value = 0.71). Eggers’ test, on the other hand, found publication bias in anti-MAP antibodies data (intercept = 1.61, 95% CI: 0.45 – 2.77, t = 2.72, p = 0.021), but not in MAP DNA dataset (intercept = -5.57, 95% CI: -20.44 – 9.29, t = -0.74, p = 0.54). The robustness of the meta-analyses was demonstrated by all sensitivity analyses. In addition, there is no evidence of p-hacking observed (right-skewness test (PFull < 0.001, PHalf <0.001; statistical power ≥ 94% (95%-CI: 72.5%-99%)). In conclusion, the synthesis revealed a strong association between MAP and MS, indicating that MAP is a significant environmental agent that may trigger MS. Thus, early screening of MAP in MS cases may assist in the therapeutic approach to its management/treatment. Therefore, future studies should be tailored towards the role of MAP in the severity of MS phenotypes, as well as address global data gaps and low disease surveillance.
COMMENTS
The idea that MAP is a cause of multiple autoimmune diseases is plausible and backed by scientific data. Because MAP is in food (dairy and meat products primarily) and domestic water supplies, most humans are regularly exposed. For more on this see the page on MAP in food and water on this website.
As the MAP epidemic in food-producing animals has expanded over the past century, so too has the frequency of autoimmune disease in humans. There are global maps of where autoimmune diseases such as MS, Crohn’s disease, Type 1 Diabetes and others occur most commonly. These maps generally coincide with similar global maps showing the prevalence of MAP infections in animals (see the page on Zoonotic Potential). Whether this is a causal or coincidental relationship remains to be firmly established or accepted by the medical community. As an interesting added bit of information supporting a causal role of MAP, Dr. C.T. Dow recently reported that “Epidemiologic evidence points to BCG (a vaccine for tuberculosis) providing a “heterologous” protective effect on assorted autoimmune diseases; studies using BCG vaccination for T1D (Type I Diabetes) and MS have shown benefit in these diseases (Microorganisms, 2020).
Sadly, despite mounting evidence that MAP affects human health in diverse ways, the problem is largely being ignored. Public health agencies such as NIH and CDC and regulatory authorities such as the FDA and USDA have not labeled MAP a food safety, water safety, or public health problem despite finding live MAP in retail foods, detecting MAP in domestic water supplies, finding MAP in Crohn’s disease patients by culture-based methods and PCR, or as immune responses to MAP infection in Type 1 Diabetes and MS.
This creates a circular problem: no acknowledgment that MAP is a human health issue results in little or no research funding and thus no definitive answers as to the ongoing question of MAP and human health. Without funding, researchers can’t unravel this potentially huge and exceedingly complex problem. Agencies funding animal health research have limited funds and do not expend those precious funds on human health research. Agencies that fund human health research do not recognize MAP as a human pathogen making it difficult for the research community to get funding to conduct the necessary research. Moreover, the problem requires a team of scientists with MAP expertise working in close collaboration with specialists in the various human diseases of concern; a One Health approach. Only if such funding bodies openly recognized MAP as a potential human health problem will One Health research teams have success at securing the necessary funding to unravel the impacts of MAP on humans and find rational solutions for the future.