Research Report
KST Kanankege and colleagues from the Department of Population Medicine, College of Veterinary Medicine, University of Minnesota reported on use of milk ELISA testing data from a voluntary testing program through DHI testing laboratories as a passive surveillance tool for Johne’s disease in Minnesota. Their report appears in the journal BMC Veterinary Research 15:429, 2019. [Open Access]
Abstract
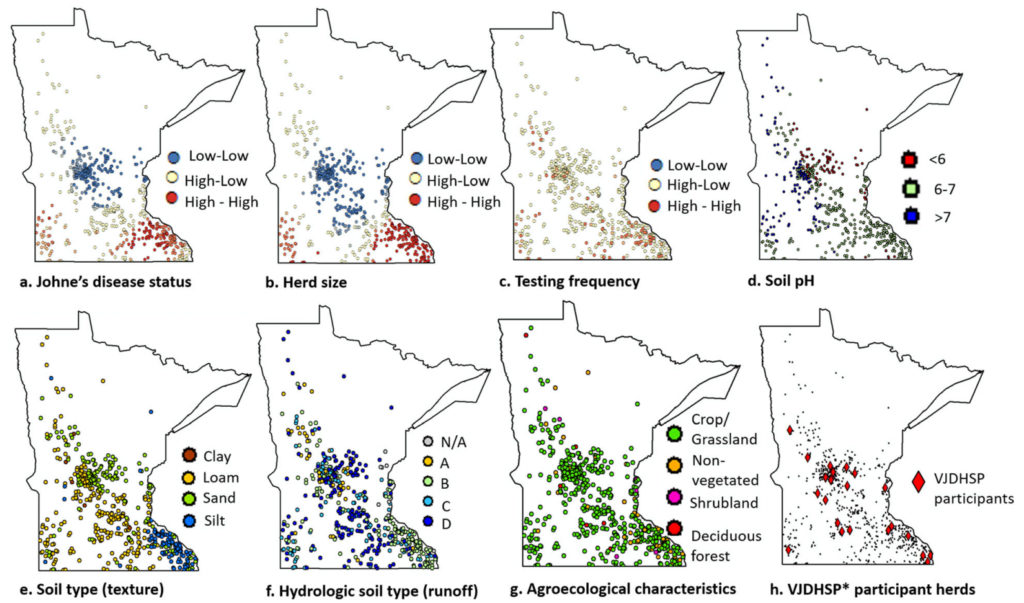
Background: One of the key steps in the management of chronic diseases in animals including Johne’s disease (JD), caused by Mycobacterium avium subsp. paratuberculosis (MAP), is the ability to track disease incidence over space and time. JD surveillance in the U.S. dairy cattle is challenging due to lack of regulatory requirements, imperfect diagnostic tests, and associated expenses, including time and labor. An alternative approach is to use voluntary testing programs. Here, data from a voluntary JD testing program, conducted by the Minnesota Dairy Herd Improvement Association, were used to: a) explore whether such a program provides representative information on JD-prevalence in Minnesota dairy herds, b) estimate JD distribution, and, c) identify herd and environmental factors associated with finding JD-positive cows. Milk samples (n = 70,809) collected from 54,652 unique cows from 600 Minnesota dairy herds between November 2014 and April 2017 were tested using a MAP antibody ELISA. Participant representativeness was assessed by comparing the number of JD-tested herds with the number of herds required to estimate the true disease prevalence per county based on official statistics from the National Agricultural Statistical Services. Multivariable logistic regression models, with and without spatial dependence between observations, were then used to investigate the association between herd status to JD (positive/negative), as indicated by milk ELISA results, and available covariates at the herd level.
Results: Within the study population, at least one test-positive cow was found in 414 of 600 (69%) herds. Results indicated that large herds that test frequently and herds located in loamy or silt soils are more likely to have at least one MAP test-positive cow. After adjusting for herd size, testing frequency, and soil type, there was no spatial dependence in JD risk between neighboring dairies within 5 to 20 km. Furthermore, the importance of collecting data on herd management, feed, and biosecurity for insightful interpretations was recognized. The study suggested that, although limited, the voluntary testing database may support monitoring JD status.
Conclusions: Results presented here help elucidate the spatial characteristics of JD in Minnesota and the study may ultimately contribute to the design and implementation of surveillance programs for the disease.
Comments: This excellent article demonstrates the power of utilizing diagnostic testing data for Johne’s disease to explore relationships and environmental factors affecting the prevalence of Johne’s disease. This is data that otherwise would provide no benefit to the dairy industry and simply sit idly on computer hard drives. Kudos to the Minnesota Dairy Herd Improvement Association for their support in data collection! Imagine if all DHI labs and all state veterinary diagnostic labs could/would share data in this same way to provide a comprehensive understanding of the spatial distribution and risk factors affecting Johne’s prevalence in the U.S.: a relatively small investment with potentially big returns.