 Dr. David Graham, Baylor College of Medicine, presented results of the Phase III randomized, double blinded, placebo controlled, multicenter, parallel group study to assess the efficacy of anti-MAP therapy (RHB-104) in the treatment of Crohn’s disease at the United European Gastroenterology (UEG) Week that took place in Vienna, October 20 – 24, 2018.
Dr. David Graham, Baylor College of Medicine, presented results of the Phase III randomized, double blinded, placebo controlled, multicenter, parallel group study to assess the efficacy of anti-MAP therapy (RHB-104) in the treatment of Crohn’s disease at the United European Gastroenterology (UEG) Week that took place in Vienna, October 20 – 24, 2018.
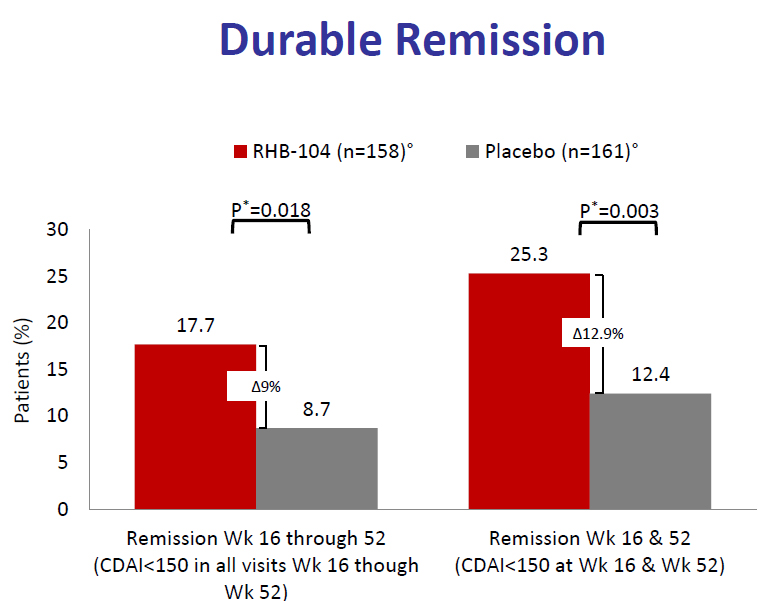
A previous press release announced significant remission levels for RHB-104-treated patients compared to controls at the 26 week time point. RedHill now announces that the remission was durable, i.e. significantly better than placebo controls at 52 weeks. This is one of the data slides shown.
This link provides the October 22 RedHill press release.
This link provides the 22 slides shown by Dr. Graham at the UEG meeting (with permission). These slides provide details on the study design, data analysis methods, and results at primary and secondary endpoints as well as results on study subgroups, e.g. comparison on patients treated with anti-TNF drugs or corticosteroids versus controls.
johnes.org is grateful to Dr. Graham and RedHill BioPharma for granting permission to provide this information to its readers.