Surveillance of cattle herds for MAP infections can be costly if individual cows are tested by fecal PCR. Some countries, such as the U.S., have adopted methods allowing pooling of fecal samples from 5 cattle before doing culture of PCR testing. If the pooled fecal sample tests PCR-negative, then all cattle contributing to that pooled sample are considered PCR-negative.
Australian researchers published a simulation modelling study indicating that the pool size can be increased to 10. Their study was published in the journal Preventive Veterinary Medicine.
ABSTRACT
Johne’s disease is a chronic intestinal disease affecting livestock. It leads to the shedding of Mycobacterium avium subspecies paratuberculosis (MAP) in the faeces, wasting and eventually death, with animal welfare, economic, and trade implications. The Johne’s Beef Assurance Scheme, used in Australia to determine the risk of Johne’s disease on beef properties and facilitate trade, is based on testing a subset of the herd with pooled faecal quantitative PCR. This study aimed to model the herd-sensitivity of pooled faecal testing under different Australian farming scenarios. Animals from simulated herds were randomly sampled and allocated into their respective pools. Each tested pool was provided a test outcome, with herd-sensitivity estimated as the probability of detecting a truly infected herd. The models simulated the test performance for the ‘Sample’ and ‘Check’ tests used in the assurance schemes (recommended sample sizes of 300 and 50, respectively) for a range of herd sizes, infection prevalence and MAP faecal shedding levels for the pool sizes of 5, 10, 15 and 20. Sensitivity and specificity input values of each pool size were obtained from a previous laboratory investigation. The herd-sensitivity estimate increased with herd size and infection prevalence levels, regardless of the pool size. Higher herd-sensitivity was also achieved for testing scenarios involving larger sample sizes. A pool size of 10 achieved similar herd-sensitivity to that of the current pool size for the majority of the Sample test and Check test scenarios. This was particularly evident when pool-specificity was assumed to be perfect. The overall herd-sensitivity of the Check test was very low for all infection prevalence levels and pool sizes, but it more than doubled, when the sample size increased from 50 to 100 animals (11% versus 26% for a herd size of 500 cattle with a 2% infection prevalence). The results show that the majority of beef producers participating in the assurance scheme can benefit from using a larger pool size for the pooled faecal quantitative PCR testing of their herd, in comparison to the pool size currently used.
COMMENTS
Kalis et al. (2000) first demonstrated that fecal sample pooling (5/pool) was effective at detecting MAP-infected dairy herds. Importantly, he showed that samples should be pooled based on animal age. He called this “strategic pooling”. The importance of this cannot be stressed enough. Animals born around the same time experience similar risks of acquiring a MAP infection. Therefore age-based (strategic) pooling helps insure that the least number of sample pools test positive and thus the fewest number of individual cattle require follow-up PCR testing.
Wells et al. (2002) compared dairy cattle fecal pools of 5 and 10 and concluded that pools of 5 were slightly more sensitive than pools of 10 when using fecal culture as the diagnostic test. This was a small study that used feces from only 10 cows to construct various fecal combinations in pools.
Van Schaik et al. (2007) collected 50 fecal samples from each of 12 Chilean dairy herds and compared pools of 5 and 10 samples finding that pools of 10 were as good or better than pools of 5 at detecting MAP-infected dairy herds.
It is challenging for veterinary diagnostic laboratories to change protocols but perhaps the time has come to consider using age-based pooling of 10 fecal samples in cattle herds that have a very low or zero rate of MAP-infections. Unless a herd is trying to meet US. or other Johne’s disease program standards for herd classification, there is no mandate to test fecal samples in pools of 5. Standards may differ in other countries.
Below are cost calculations for a herd of 100 cattle tested by fecal PCR using pools of 5 or 10 cattle/pool. Only laboratory fees are calculated, and prices are based on the rates posted on the Wisconsin Veterinary Diagnostic Laboratory (WVDL) website 26-MAR-2021 (individual feal PCR @ $30.89 and pooled PCR @ $35.02). It does not include the $10 accession fee, veterinary costs, or sample shipping. Costs are rounded to the nearest U.S. dollar. Note: The WVDL has no extra cost for Johne’s disease tests for samples originating from states or than Wisconsin.
For comparison, the cost of testing 100 cattle individually by fecal PCR would be $3,502.
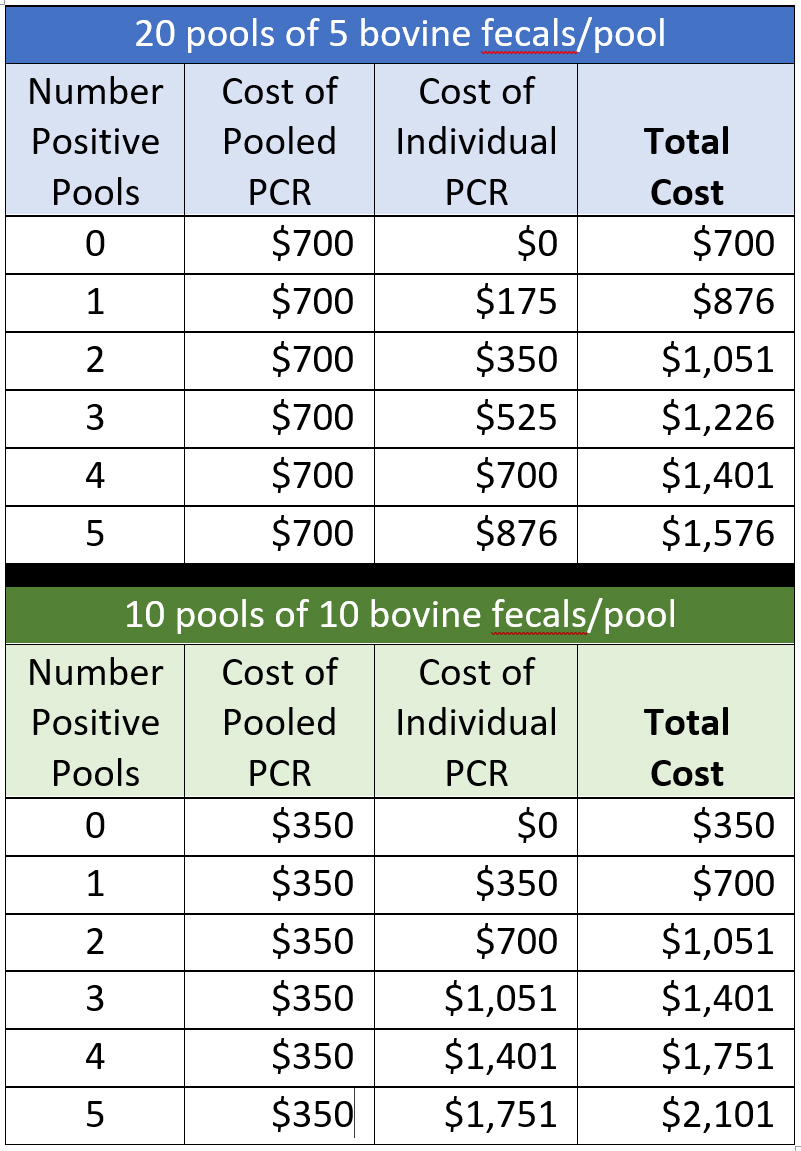
This table illustrates that:
- Sample pooling can save money. Even if 5 fecal pools test PCR-positive the total cost for testing a herd of 100 cows would be less than testing each cow individually by fecal PCR.
- Sample pooling is most advantageous for herds with a low MAP infection prevalence, i.e., few PCR-positive pools.
It is important to stress that to have the fewest number of positive pools, samples should be pooled based on animal age. This will tend to group fecal samples from MAP-infected cows into the same sample pool results in in fewer pools requiring individual fecal sample testing.
How does this compare to testing by ELISA (blood sample testing)?
The cost for testing a herd of 100 cows by ELISA would be $600 at current WVDL prices. In most instances, cows testing ELISA-positive would have to be retested by individual fecal PCR to confirm the diagnosis. This makes the per head cost of doing pooled fecal PCR equal to or less than that of testing by ELISA for low prevalence herds. This is especially true if pools of 10 are tested. Given that the fecal PCR is roughly 2 to 3 times more sensitive than the ELISA, herd owners get a better return on their investment in Johne’s disease diagnostics by using fecal PCR on pooled samples; no need to resample and test ELISA-positive cows by fecal PCR and greater confidence in that the PCR-negative cattle are truly not MAP-infected.
Not all laboratories offer pooled fecal PCR testing and prices vary considerably among veterinary diagnostic laboratories. Talk to your herd veterinarians and consult with testing laboratories before sending samples. This website provides a list of laboratories that are USDA-approved to perform pooled fecal testing: scroll down to the PDF file named “Johne’s Disease-pooling methods”.
What about sheep and goats?
Sample pooling studies have not been reported for goats, but cattle-based studies are a good guide. In Australia, fecal samples from 50 sheep are pooled before PCR testing.