SHEEP AND SAMPLE POOLING
2020-01-05 17:33:52Research Report
Mathevon and colleagues from Ecole Nationale Vétérinaire de Toulouse, Toulouse Cedex, France reported on optimal sheep sample pooling strategies for serum samples (for ELISA testing) or fecal samples (for qPCR testing). Their report appeared December 26, 2019 in PLoS ONE and is OPEN ACCESS. [18 pages with 47 references]
Abstract
The aim of our study was to evaluate the flock sensitivity and specificity of fecal qPCR and serum ELISA using pooled samples for screening paratuberculosis in French sheep.
Using individual feces with low or high qPCR Ct values from ewes sampled in 14 infected flocks, a total of 555 pools of size 5, 10 and 20 were created by diluting individual materials in negative feces and analysed using a commercial IS900 qPCR kit. The relative performances of pooled serum ELISA analysis were evaluated based on the analysis of 181 different pools of size 5 and 10, composed of individual serum samples of various individual S/P values. Results showed that for pools of size 5, 10 or 20, individual fecal samples with low Ct values were invariably detected. Conversely fecal samples with high Ct values were associated with a lower detection rate in both pools of size 5 (87.0% to 90.0%), 10 (63.0% to 70.7%) and 20 (46.7% to 60.0%). After lowering the decision threshold to 25% and 15% for serum pools of size 5 and 10 respectively, the pooled serum ELISA relative sensitivity ranged between 62.2% and 100.0% depending on the composition of the pools.
Finally, a simulation study was carried out to evaluate the performances of 16 screening strategies at flock level, with varying pool size (5 to 20) and number (5 to 60). The use of pooled serum ELISA led to very false positive detection rate ranging between 37.6% and 91.8% in paratuberculosis free flocks and prevents its further use in that context. For infection prevalence ≤ 5%, the flock sensitivity based on pooled fecal qPCR ranged between 39.0% (5 pools of size 10) and 99.9% (300 sampled individuals, with pools of size 5,10 or20), and was always above 93% when the infection prevalence was greater or equal to 15%. We conclude that pooled-fecal qPCR but not pooled-serum ELISA could be a useful tool to detect sheep flocks infected with paratuberculosis.
Comment: The authors focused their discussion on diagnostic tests useful for screening of sheep flocks for ovine Johne’s disease (OJD) where the primary goal is to detect MAP-infected flocks, i.e. maximize “flock sensitivity” and “flock specificity”, at the least cost. This should not be confused with the use of fecal pooling strategies for qPCR testing in known infected flocks where the goal is to identify individuals in the flock that should be culled or isolated.
In MAP-infected flocks there are two equally important goals: 1) to limit the cost of testing the whole flock, and 2) to limit the cost of testing the individual samples, i.e. to have the fewest number of qPCR-positive pools. This second goal is important because each of the fecal samples comprising a qPCR-positive pool must be individually tested by qPCR in order to identify each MAP-infected sheep adding significant costs, e.g. roughly $30 x 5 = $150 per PCR-positive pool (based on WVDL testing rates).
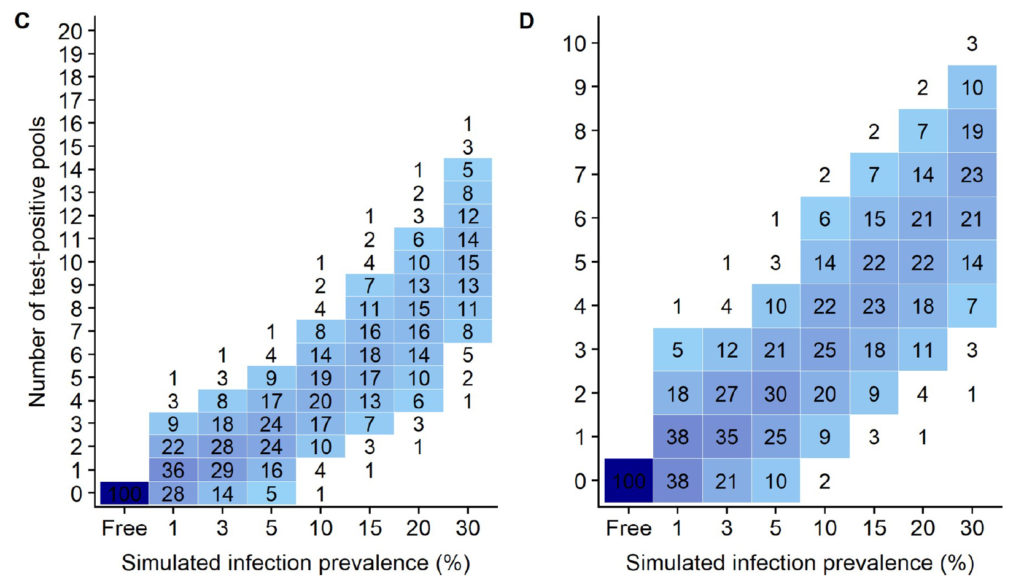
Using the simulation data from this publication (shown above), for flocks with a MAP infection prevalence of 10%, the simulation data (Fig 4 C & D in the publication as shown above) indicate that using 20 pools of 5 fecals/pool, 93% of flocks would have 7 or fewer positive pools which would then cost an additional $1,050 to test each fecal individually by qPCR (7 pools x 5 fecals/pool x $30/qPCR = $1,050). By contrast, if these same flocks used 10 pools of 10 fecals/pool, then 92% of flocks would have 5 or fewer qPCR-positive pools resulting in a cost of $1,500 to individually test the fecal samples making up those pools (5 pools x 10 fecals/pool x $30 = $1,500).
The cost of testing by pooled PCR at the WVDL is $34/pool so would cost $340 for 10 pools of 10 fecals/pool or $680 for 20 pools of 5 fecals/pool. Thus, while testing 20 pools (5 fecals/pool) would initially cost $340 more, the cost of testing the individual fecals comprising each qPCR-positive pool does not offset the cost of testing fewer pools in this example.
These findings are based on a simulation of random pooling of fecal samples described in the publication. As mentioned in a previous news posting, strategic pooling by animal age can help limit the number of test-positive pools Kalis (2000). This is because animals of similar age experienced similar risks of becoming MAP-infected when young. Thus, age-based pooling (strategic) results in more MAP-infected animals likely to be in the same pool and conversely the fewest number of positive pools for the flock or herd as a whole.
You can read more about a clustering of MAP-infections by age and season in the publication by Zare et al. titled: Evidence of birth seasonality and clustering of Mycobacterium avium subspecies paratuberculosis infection in US dairy herds. Preventive Veterinary Medicine 112: 276– 284, 2013. However, the clustering effect may not be as pronounced for sheep which do not have lambs thought the year.
In closing, this discussion highlights the importance of utility analysis when judging the merits of diagnostic tests for MAP. For more on this topic see Garder et al. Consensus-based reporting standards for diagnostic test accuracy studies for paratuberculosis in ruminants. Preventive Veterinary Medicine 101: 18-34, 2011.
MAP IN BRAZILIAN CHEESE
2019-12-30 15:39:49Research Report
Brazilian researchers report a small survey of a popular cheese eaten in Brazil for MAP. Their findings were reported in Arquivo Brasileiro de Medicina Veterinária e Zootecmia (vol.71 no.6 Belo Horizonte Nov./Dec. 2019 Epub Dec 13, 2019).
Abstract
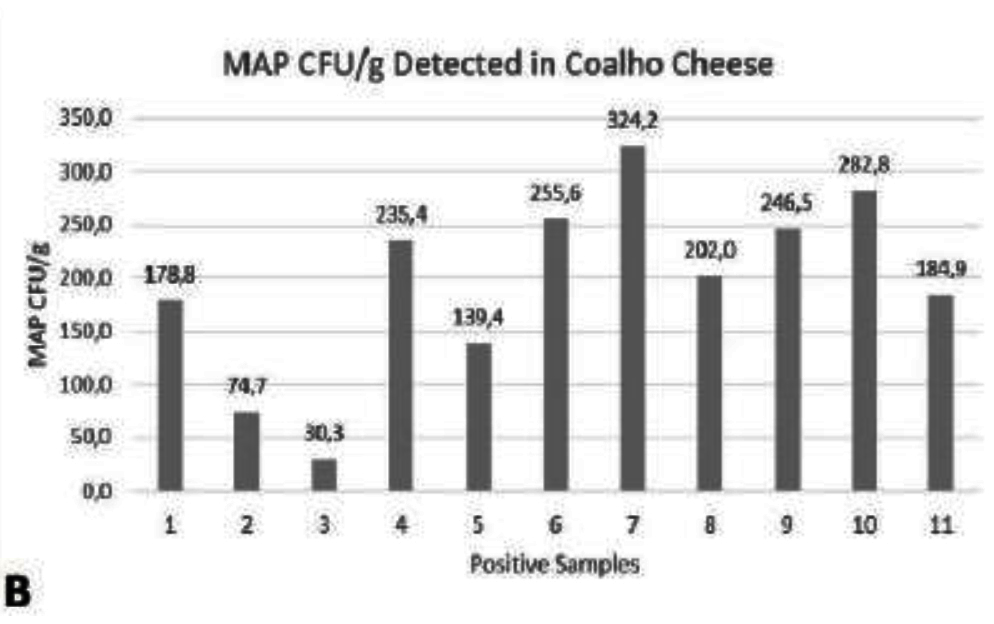
Paratuberculosis is a chronic and incurable disease that affects ruminants and other domestic animals. It is caused by Mycobacterium avium subsp. paratuberculosis (MAP) that may also be involved in some human diseases such as Crohn's disease, type 1 diabetes, sarcoidosis, multiple sclerosis, and Hashimoto's thyroiditis. The objective of this study was to investigate the occurrence of MAP DNA in samples of artisanal coalho cheese purchased in the State of Pernambuco. Forty samples of coalho cheese submitted to the Real Time Polymerase Chain Reaction (qPCR) technique were analyzed for the detection of the MAP region IS900. 11 (27.5%) were positive with a mean of 195.9 MAP colony forming unit (CFU) per gram of each sample, with a minimum of 30.3 CFU/g and a maximum of 324.2 CFU/g. Thus, this type of cheese that is one of the most consumed in this region of Brazil constitutes a source of human exposure to MAP. Further research in this area should be performed to evaluate the viability of the bacteria in this cheese type.
Comment: It should be noted that MAP numbers (CFU) were actually based 10-fold serially diluted MAP DNA used to obtain the standard curve, in which it was possible to detect from 21 up to 2.12 x 107 MAP cells. In my opinion a more informative titration curve (MAP CFU vs qPCR Ct values) would begin with known CFU/mL MAP suspensions that were then processed by DNA extraction followed by qPCR. Only then can an investigator truly know the net results of both DNA extraction and target IS900) amplification to estimate the CFU of MAP in the original sample. That said, most molecular biologists opt for titration of extracted DNA as the authors of this study did.
The last line of the abstract is the critical one. The really big question is: Are the detected MAP alive or dead. That said, some investigators postulate that dead MAP may function as a dietary allergen triggering inflammation in the absence of infection. MAP as a food safety threat is a critical knowledge gap urgently requiring research.
MAPPING MAP IN MINNESOTA
2019-12-23 13:04:54Research Report
KST Kanankege and colleagues from the Department of Population Medicine, College of Veterinary Medicine, University of Minnesota reported on use of milk ELISA testing data from a voluntary testing program through DHI testing laboratories as a passive surveillance tool for Johne’s disease in Minnesota. Their report appears in the journal BMC Veterinary Research 15:429, 2019. [Open Access]
Abstract
Background: One of the key steps in the management of chronic diseases in animals including Johne’s disease (JD), caused by Mycobacterium avium subsp. paratuberculosis (MAP), is the ability to track disease incidence over space and time. JD surveillance in the U.S. dairy cattle is challenging due to lack of regulatory requirements, imperfect diagnostic tests, and associated expenses, including time and labor. An alternative approach is to use voluntary testing programs. Here, data from a voluntary JD testing program, conducted by the Minnesota Dairy Herd Improvement Association, were used to: a) explore whether such a program provides representative information on JD-prevalence in Minnesota dairy herds, b) estimate JD distribution, and, c) identify herd and environmental factors associated with finding JD-positive cows. Milk samples (n = 70,809) collected from 54,652 unique cows from 600 Minnesota dairy herds between November 2014 and April 2017 were tested using a MAP antibody ELISA. Participant representativeness was assessed by comparing the number of JD-tested herds with the number of herds required to estimate the true disease prevalence per county based on official statistics from the National Agricultural Statistical Services. Multivariable logistic regression models, with and without spatial dependence between observations, were then used to investigate the association between herd status to JD (positive/negative), as indicated by milk ELISA results, and available covariates at the herd level.
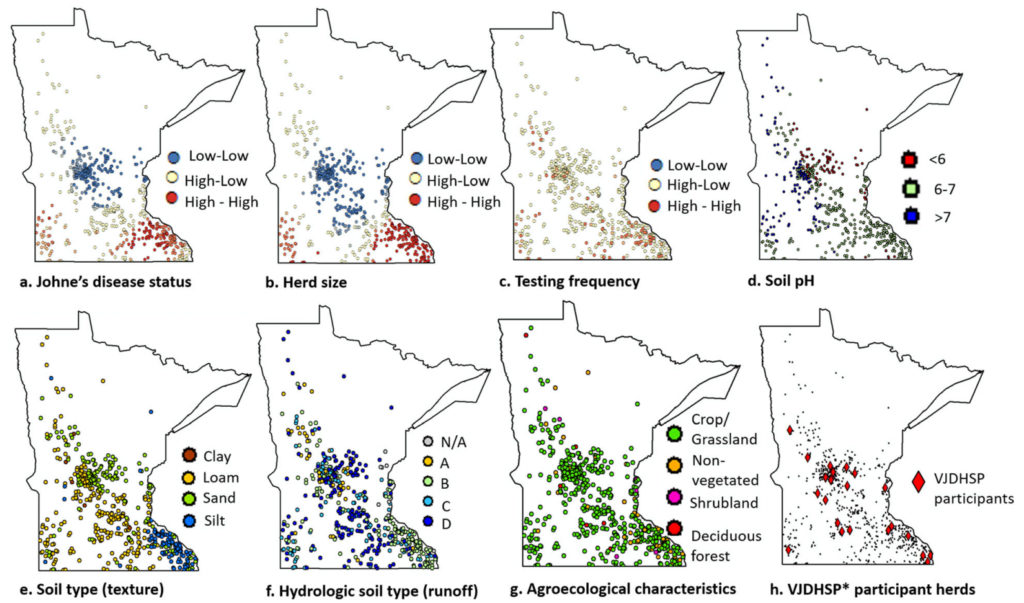
Results: Within the study population, at least one test-positive cow was found in 414 of 600 (69%) herds. Results indicated that large herds that test frequently and herds located in loamy or silt soils are more likely to have at least one MAP test-positive cow. After adjusting for herd size, testing frequency, and soil type, there was no spatial dependence in JD risk between neighboring dairies within 5 to 20 km. Furthermore, the importance of collecting data on herd management, feed, and biosecurity for insightful interpretations was recognized. The study suggested that, although limited, the voluntary testing database may support monitoring JD status.
Conclusions: Results presented here help elucidate the spatial characteristics of JD in Minnesota and the study may ultimately contribute to the design and implementation of surveillance programs for the disease.
Comments: This excellent article demonstrates the power of utilizing diagnostic testing data for Johne’s disease to explore relationships and environmental factors affecting the prevalence of Johne’s disease. This is data that otherwise would provide no benefit to the dairy industry and simply sit idly on computer hard drives. Kudos to the Minnesota Dairy Herd Improvement Association for their support in data collection! Imagine if all DHI labs and all state veterinary diagnostic labs could/would share data in this same way to provide a comprehensive understanding of the spatial distribution and risk factors affecting Johne’s prevalence in the U.S.: a relatively small investment with potentially big returns.
OPTIMAL FECAL POOL SIZE
2019-12-15 18:44:50Research Article
Australian veterinary scientists report studies to define the optimal number of fecal samples from beef cattle that can be pooled for MAP detection by fecal PCR. Their findings were published in PLoS ONE and the article is Open Access [18 pages with 42 references]. This was mentioned in last weeks news posting but I feel it deserves more explanation.

Abstract
Bovine Johne’s disease (JD) is a chronic debilitating disease affecting cattle breeds worldwide. Pooled faecal samples are routinely tested by culture to detect Mycobacterium avium subsp. paratuberculosis (Mptb) [also called MAP] infection. More recently, a direct high throughput molecular test has been introduced in Australia for the detection of Mptb in faeces to circumvent the long culture times, however, the optimal pool size for beef cattle faeces is not known. This study aimed to determine the optimal pool size to achieve the highest test sensitivity and specificity for beef cattle. Individual archived faecal samples with low, medium and high quantities of Mptb (n = 30) were pooled with faecal samples from confirmed JD negative animals to create pool sizes of 5, 10, 15 and 20, to assess the diagnostic sensitivity relative to individual faecal qPCR. Samples from JD-free cattle (n = 10) were similarly evaluated for diagnostic specificity. Overall, 160 pools were created, with Mptb DNA extracted using magnetic bead isolation method prior to Mptb-specific IS900 quantitative PCR (qPCR). The pool size of 10 yielded the highest sensitivity 73% (95% CI: 54–88%), regardless of the quantity of Mptb DNA present in the faeces. There was no significant differences between the four different pool sizes for positive pool detection, however, there was statistical significance between low, medium and high quantities of Mptb. Diagnostic specificity was determined to be 100%. The increase in pool size greater than 10 increased the chances of PCR inhibition, which was successfully relieved with the process of DNA dilution. The results of this study demonstrate that the pool size of 10 performed optimally in the direct faecal qPCR. The results from this study can be applied in future simulation modelling studies to provide suggestions on the cost-effective testing for JD in beef cattle.
Comment: Dr. Kees Kalis (2000) also showed that pooling of 5 fecal samples was effective when using culture as the diagnostic test. Kalis also noted that “strategic pooling” can further enhance the procedure. Strategic polling refers to grouping animals of the same or similar ages into the same pools.
A study on fecal sample pooling by Wells et. al. (2002) using artificially constructed pools of feces from dairy cattle showed that 5 samples per pool was only slightly better than 10 samples per pool, when using culture of MAP on solid media as the diagnostic test. The standard protocol for sample pooling in the U.S. has remained at 5 per pool ever since.
The Australian study used a much larger number of samples, they originated from naturally infected beef cattle, and the diagnostic assay was the high throughput PCR (Plain, 2014). The ability to pool 10 samples instead of 5 obviously decreases the laboratory costs for the producer by half with apparently little or no loss in diagnostic accuracy. If widely adopted, this would encourage more testing and verification of cattle herds that are not MAP-infected which then would be preferred sources of replacement cattle. These cattle could sell at premium prices thus allowing the herd owner to recoup the costs of attaining a test-negative status.
Australia has been using fecal sample pooling for sheep since 2000 finding that fecal pellets from 50 sheep can be combined into a single pooled test without significant loss in MAP infection detection at the flock level (Whittington, 2000).
Due to lack of funding, few cattle herds currently participate in the US. JD program (formally called the Voluntary Johne’s Disease Herd Status Program (VJDHSP). Thus, for herd owners simply wanting the least cost JD surveillance program, without concern for formal USDA recognition of the herd JD status, then it makes sense to use the 10 fecals/pool testing scheme.
Lastly, samples should be carefully collected, packaged and shipped refrigerated to the lab. The containers should seal tightly to prevent leakage. Below is an example of how NOT to send fecal samples. These snap-top containers were filled too full and not refrigerated causing gas to be produced popping the tops off the containers making for a poopy mess and cross-contamination of samples rendering them useless. The photo above shows well-collected and labeled bovine fecal samples in Whirlpack bags.

TESTING BEEF CATTLE HERDS
2019-12-12 14:51:33John Campbell published an article in the December 6 issue of The Western Producer titled: Testing beef herds for Johne’s disease can be difficult. Interestingly, he begins his article stating: "Every year, I seem to write another article about Johne’s disease. It is one of the most common topics of phone calls and questions that I receive from veterinarians and producers."
It’s very helpful that this article written for beef cattle producers calls attention to Johne’s disease. However, it is more pessimistic about diagnostic testing than necessary.
This news posting offers an alternative perspective.
Mr. Campbell describes a hypothetical 1,000 cow herd with a 10% true MAP infection rate. He analyzes what would occur using a blood test (ELISA) with a sensitivity and specificity of 30% and 99%, respectively. He explains that on a single herd test there will be too many false-negative test (7 of the 10 MAP-infected cows would be missed). In his example, he claims there also would be too many false-positive test results (9 cows that are truly not MAP-infected would test positive) resulting in too many mistakenly culled cows. There are several problems with this analysis.
First, the beef cattle herds that should be testing are those sell breeding stock, often called seedstock producers. For commercial cow-calf producers a such a testing program is good for animal health and welfare but hard to justify economically. For seedstock producers, eradicating MAP infections or proving herds are not MAP-infected must be a business objective to avoid spreading MAP infections to other herds.
Second, beef seedstock herds should not be using a blood test (ELISA). Rather they should use a test with higher sensitivity and specificity namely the direct fecal PCR assay. The high throughput PCR assay used in Australia has a reported sensitivity and specificity of 60.4% and 99.6%, respectively (Plain, 2014).
Also, ELISAs are screening tests and most experts recommend doing a confirmatory test (culture or PCR) on all ELISA-positive animals before making a decision to cull the cow. Especially if the cow is valuable.
Had Mr. Campbell used fecal PCR as the test in his example, only 4 of the 10 MAP-infected cows would be missed on a single whole herd test and these so called false-negative cows would be animals that were shedding few or no MAP in their feces, meaning they are not infectious. These animals would likely be detected in the next annual herd test. The false-positive rate would be 0.4% (1.00 minus specificity) or 3 - 4 cows (890 non-infected cows x 0.4% = 3.5).
Not only is a direct fecal PCR the test of choice, but this assay is more economical because it can be done on pooled fecal samples (the lab does the pooling). Until recently, the standard pooling method mixed fecal samples from 5 cows into a single pool that was then tested by PCR. This month, Australian researchers showed that this can be increased to 10 animal per pool (Ly, 2019).
At the Wisconsin Veterinary Diagnostic Laboratory the ELISA cost is $6/sample and the pooled PCR is $34/pool, or $6.80/cow, using pools of 5, and it would be only $3.40/cow, using pools of 10. Some additional costs would be incurred to run a confirmatory test on ELISA-positive cows and to test individual cows in the PCR-positive fecal pools.
____________
In summary, pooled fecal PCR is the test of choice for beef cattle seedstock herds. This assay provides twice the diagnostic sensitivity (rate of detecting MAP-infected cows) and higher diagnostic specificity (fewest false-positives) at a cost that is comparable to or lower than that of the ELISA blood test. Additionally, the fecal PCR provides numerical results that indicate which cows are shedding the most MAP, i.e. those that are most infectious and should be culled first. This is also true for breeders of sheep, goats, dairy cows and other ruminants.
ENVIRONMENTAL TESTING FOR MAP ON LARGE DAIRIES
2019-12-08 18:27:01Research Report
Chamchoy T, Williams DR, Adaska JM, Anderson RJ, Aly SS. 2019. Environmental sampling to assess the bioburden of Mycobacterium avium subspecies paratuberculosis in drylot pens on California dairies. PeerJ 7:e8081 https://doi.org/10.7717/peerj.8081

Abstract
Mycobacterium avium subspecies paratuberculosis (MAP) is a bacterium that can cause substantial economic losses in infected dairy herds due to reduced milk production and increased cow-replacement costs. In order to control MAP in dairies with drylot pens, a standardized environmental sampling protocol to quantify MAP in fecal slurry was developed based on an existing protocol for freestall pens. Specifically, following a 24 h hold of the flush, a grab sample of approximately 10 ml of fecal slurry was collected every 1 m along the flush lane of the drylot pens, avoiding individual cow fecal pats. To determine the reliability and repeatability of the new environmental sampling protocol for estimation of MAP bioburden at the pen level, two collectors simultaneously collected fecal slurry samples every day for 3 days from six drylot cow pens on two Central California dairies. During the study period no cow movement between pens was allowed with the exception of sick cows. The study herds had MAP seroprevalence of 5.8% and 3.2%, respectively, based on whole pen serum ELISA results. Variance components models for quantitative real-time PCR (qPCR) results showed samples collected from different pens on different dairies accounted for greater variability in MAP concentration (65%), while samples collected by different collectors had the least variability (0.1%). In contrast, variability in MAP concentration in environmental samples collected on different days had 25% variability. The intraclass correlation coefficient showed high reliability (93%) of environmental sampling simultaneously by different collectors. In contrast, the reliability of environmental sampling at different days was 65%, which was similar to the reliability for sampling by different collectors on different days. Investigators can expect high reliability when employing the new environmental sampling protocol along with qPCR testing of environmental samples from drylot pens.
Comment: This excellent article describes the critical variables to consider when designing surveillance programs to detect MAP infections in large drylot dairies of the type found in California, USA. The publication provides excellent details regarding the environmental sampling protocol for those interested. It is also Open Access.
NEW MAP DETECTION DIAGNOSTIC
2019-12-04 20:56:55Research Report – OPEN ACCESS
B. Swift from the department of Pathobiology and Population Sciences, Royal Veterinary College together with colleagues from the University of Nottingham and Moredun Research Institute report on a means of enhancing diagnostic sensitivity for MAP detection in blood samples by use of a bacteriophge to improve the efficiency of DNA extraction prior to performing PCR. This publication appears in the November issue of Microbial Biotechnology. PBD Biotech Ltd is the company commercializing the Actiphage® technology.
Summary
Here, we describe the development of a method that exploits bacteriophage D29 as a lysis agent for efficient DNA extraction from low numbers of mycobacterial cells. This method (Actiphage®) used in combination with PCR achieved rapid and sensitive (LOD ≤ 10 cell per ml) detection and identification of viable, pathogenic mycobacteria in blood samples within 6 h. We demonstrate that mycobacteriophage D29 can be used to detect a range of mycobacteria from clinical blood samples including both Mycobacterium tuberculosis complex and Mycobacterium avium subsp. paratuberculosis without the need for culture and confirms our earlier observations that a low-level bacteraemia is associated with these infections in cattle. In a study of M. bovis-infected cattle (n = 41), the sensitivity of the Actiphage® method was 95 % (95 % CI; 0.84–0.99) and specificity was 100 % (95% CI; 0.92–1). We further used Actiphage® to demonstrate viable Mycobacterium avium subsp. paratuberculosis is present in the blood of Johne’s infected cattle. This method provides a revolutionary new tool for the study of infections caused by these difficult to grow pathogens.
For more information contact:
Bob Lyons, GM/US, PBD Biotech, Pasadena, CA,
e: Bob@PBDBio.com p: 626 394 9771.
Comment: The diagnostic sensitivity of PCR assays is heavily dependent on the efficiency of methods to extract DNA from MAP with its thick, tough waxy cell wall. Using bacteriophages to lyse the MAP appears to be more efficient than chemical extraction methods and also allows detection of only live MAP cells since the phage will not bind to and then lyse dead MAP bacteria. Hopefully further studies by independent investigators will verify the enhanced diagnostic sensitivity of the Actiphage® system and the ability of this novel diagnostic assay to detect MAP in blood samples early in the course of a MAP infection.
JOHNE’S IN WHITE-TAILED DEER
2019-12-02 17:25:28Research Report
MV Palmer and colleagues from the U.S. National Veterinary Services Laboratory (NVSL) reported a detailed investigation of Johne’s disease in a captive white-tailed deer herd. The article appears in the Journal of Veterinary Diagnostic Investigation 31(6):844–851, 2019.
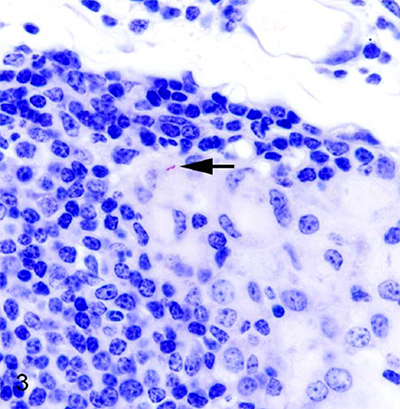
So few MAP but so much inflammation!
Abstract
Paratuberculosis (Johne’s disease) is caused by Mycobacterium avium ssp. paratuberculosis (MAP), and affects both domestic and wild ruminants, including cattle, goats, sheep, and deer. In cattle, most infections occur during calfhood followed by a prolonged incubation period of 1–2 y or more before cows shed culturable numbers of MAP bacilli in their feces. As disease progresses, infected animals develop protein-losing enteropathy, intractable diarrhea, and weight loss. In a cohort of 32 clinically normal deer from a herd with a history of periodic clinical paratuberculosis, we found that subclinical infection was characterized by high rates of infection, common involvement of mesenteric lymph nodes, minimal lesion formation, few intralesional acid-fast bacilli, and low-level fecal shedding of MAP. The characteristics of subclinical paratuberculosis in white-tailed deer resemble those of cattle and red deer, although microscopic lesions were less common in subclinical deer than reported for subclinical cattle, and we did not see necrotizing granulomas as described in subclinical red deer and elk.
Comment: This report describes a very thorough investigation with repeated sampling and extensive examination of tissues after necropsy. The photomicrographs are particularly nice and highlight how hard it is to find MAP in tissue sections, even with special stains. Unfortunately, this article is not Open Access.
MULTIPLEX MAP PCR AT THE WVDL
2019-11-25 14:30:36Announcement from the Wisconsin Veterinary Diagnostic Laboratory (WVDL)
 The WVDL has completed the validation of new multiplex real-time PCR assay for the detection of Mycobacterium avium ss. paratuberculosis (MAP) in accordance with the American Association of Veterinary Laboratory Diagnosticians (AAVLD) guidelines. WVDL has improved the specificity and sensitivity of the assay for all species routinely tested including for bovine, caprine, ovine and exotic samples. This multiplex real-time PCR assay uses three targets, which minimizes the possibility of cross-reaction with other Mycobacterium avium complex members thereby increasing MAP detection specificity. The validation data showed that only a very a small number of exotic samples (< 0.01%) cannot be resolved using the multiplex PCR. In those situations, the WVDL reports the MAP PCR result as “undetermined”, with the additional recommendation of retesting the animal within the next 6 months. If you have any questions, please feel free to contact the Microbiology Molecular Section at the WVDL by phone at 608-262-5432, by email at info@wvdl.wisc.edu or at our website.
The WVDL has completed the validation of new multiplex real-time PCR assay for the detection of Mycobacterium avium ss. paratuberculosis (MAP) in accordance with the American Association of Veterinary Laboratory Diagnosticians (AAVLD) guidelines. WVDL has improved the specificity and sensitivity of the assay for all species routinely tested including for bovine, caprine, ovine and exotic samples. This multiplex real-time PCR assay uses three targets, which minimizes the possibility of cross-reaction with other Mycobacterium avium complex members thereby increasing MAP detection specificity. The validation data showed that only a very a small number of exotic samples (< 0.01%) cannot be resolved using the multiplex PCR. In those situations, the WVDL reports the MAP PCR result as “undetermined”, with the additional recommendation of retesting the animal within the next 6 months. If you have any questions, please feel free to contact the Microbiology Molecular Section at the WVDL by phone at 608-262-5432, by email at info@wvdl.wisc.edu or at our website.
Comment: Multiplex PCR assays are not new, but this is among the first reports that the assay has been validated and made routine in a veterinary diagnostic laboratory.
I have been involved in two situations where a commonly used commercial MAP PCR kit reported a positive result on zoological ruminants only to discover after more extensive testing that the animals had a strain of M. avium subsp. avium in their fecal sample that caused a false-positive MAP PCR kit result. The 3-target multiplex has been proven to avoid this potentially costly mistake. When a MAP diagnosis by conventional real-time PCR kit has large economic consequences, it is advisable to “get a second opinion” using this multiplex PCR assay, or simply start with this multiplex assay given that the cost is comparable to what most labs charge using the single genetic target PCR kits for MAP.
____________
For the very interested, here are some research reports on multiplex PCR assays for MAP:
- DV Cousins et al. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS 900-like sequences detectable by IS 900 polymerase chain reaction: implications for diagnosis. Molecular and Cellular Probes 13(6):431-442, 1999.
- T Tasara et al. Development and evaluation of a Mycobacterium avium subspecies paratuberculosis (MAP) specific multiplex assay. International Journal of Food Microbiology 104(3):279-287, 2005.
- S Rajeev. Evaluation of multiple genomic targets for identification and confirmation of Mycobacterium avium subsp. paratuberculosis isolates using real-time PCR. Veterinary Microbiology 105(3-4):215-221, 2005.
- D Herthnek and G Bölske. New PCR systems to confirm real-time PCR detection of Mycobacterium avium subsp. paratuberculosis. BMC Microbiology 6: article no. 87, 2006.
- SJ Shin et al. Efficient differentiation of Mycobacterium avium complex species and subspecies by use of five-target multiplex PCR. Journal of Clinical Microbiology 48(11):4057-4062, 2010.
AORTIC CALCIFICATION WITH JOHNE’S DISEASE
2019-11-18 00:46:45Everyday there is something interesting I learn about Johne's disease and it comes from diverse sources. This story is about -
comparative pathology
and
collaborative learning
The email below was received last week from Dr. Fritz Schumann, a Canadian veterinarian working in Saskatoon (see map):
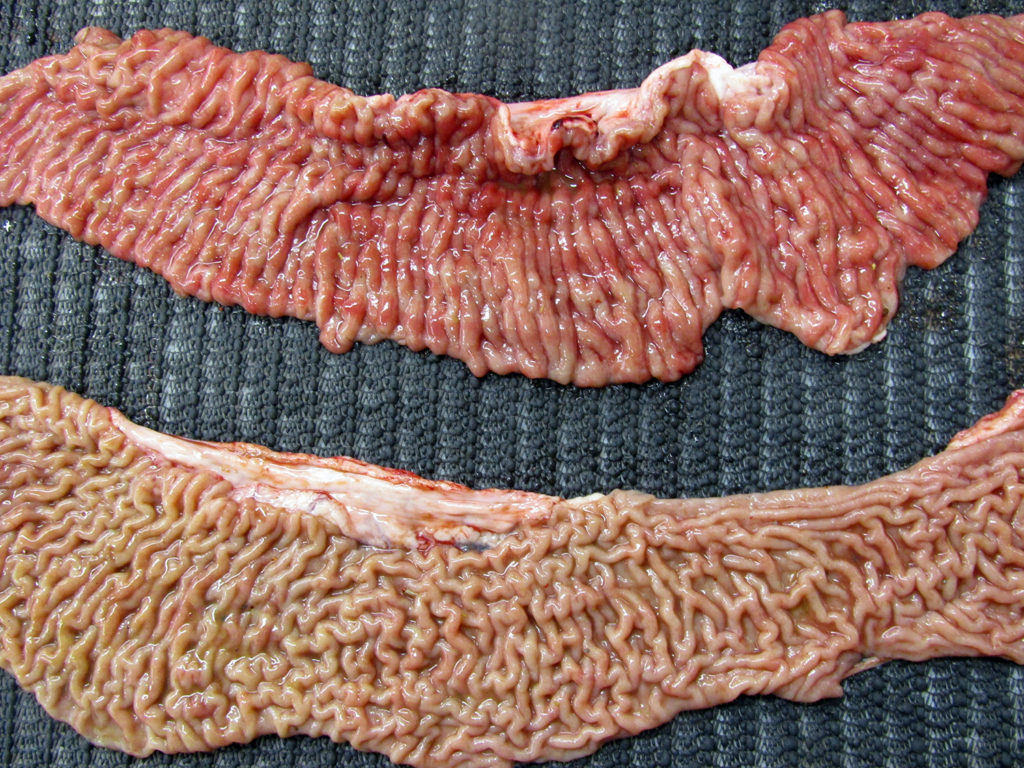
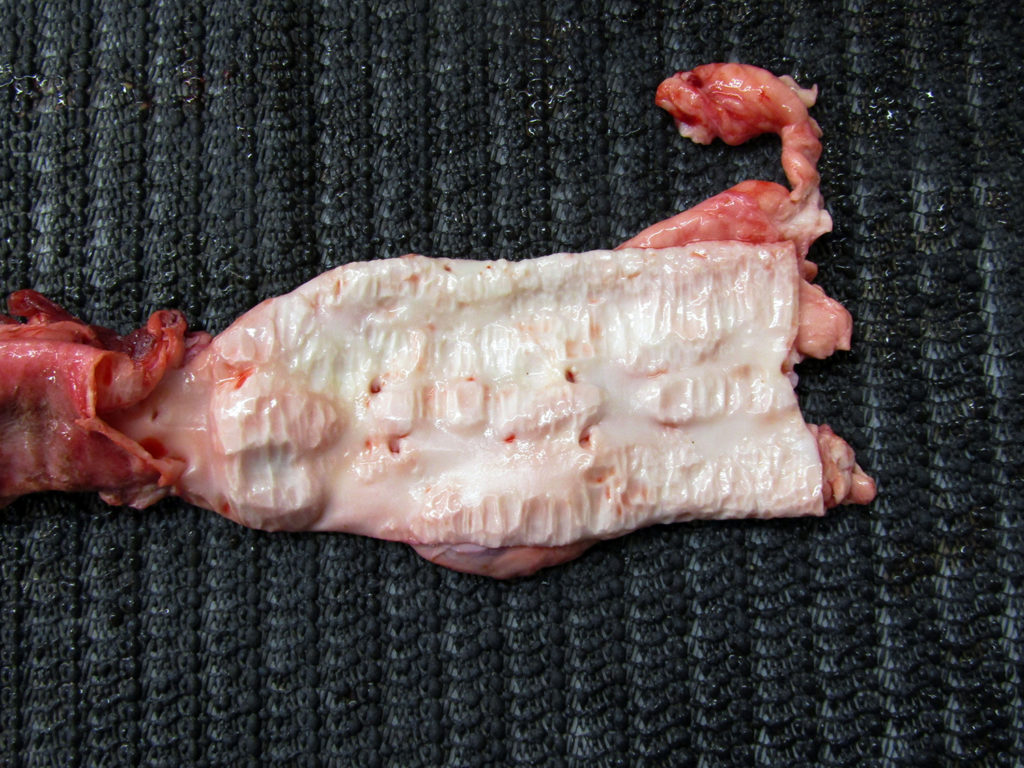
He wrote: I am vet in Saskatoon. I had this 2 year and 9-month-old Black Angus cow which I euthanized as she had Johne’s. Positive ELISA in blood and PCR positive on feces. I euthanized her and am sending two pictures. The first is her ileum and jejunum. The second picture is the aorta, looks like calcification from the aortic valve all the way to the pelvis then I stopped looking. The aorta felt like there is no elastic tissue left and paper thin. My question is why does aorta change like that with Johne’s? This is the worst aorta I have seen. Thank you very much for reading my email and considering my request for an explanation.
THANK YOU DR. SCHUMANN! He granted me permission to show his exceptional pictures taken at the Western College of Veterinary Medicine. The first shows the classic thickened ileum (top) and the jejunum for comparison (bottom).
This second photo shows the striking degree of calcification of the aorta.
I made inquiries with colleagues and this reply came from the Minnesota Veterinary Diagnostic Laboratory pathologists. Below is their quick and informative reply. Big thanks! to these pathologists.
Provided here is an excerpt from a paper that was published in JVDI on blastomycosis in a llama (J Vet Diagn Invest. 2018 Jul; 30(4): 576–579). It provides some thoughts on possible pathogenesis:
"As a component of chronic disease, monocytes and macrophages can generate and release tumor necrosis factor–alpha (TNFα) that drives mineralization, and macrophages may accumulate basic calcium phosphate that can locally increase cytokine production (TNFα, interleukin [IL]-1, and IL-8) that further drives mineralization and stimulates endothelial cells to differentiate toward osteoblasts (20). In dromedary camelids and cattle with Johne’s disease, which exhibits a predominantly granulomatous cellular response, proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-10, interferon-γ, and TNF-α), acute-phase and oxidative-stress proteins are significantly increased (9). Hypercalcemia has been reported in dogs with granulomatous diseases, including blastomycosis, and is likely associated with the conversion of calcifediol (25-hydroxyvitamin D) to calcitriol (1,25-dihydroxyvitamin D) by activated macrophages (8,14)."
Citations mentioned in this 2018 JVDI excerpt:
- #8. Dow SW, et al. Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc 1986;188:706–709.
- #9. El-Deeb W, et al. Clinico-biochemical investigation of paratuberculosis of dromedary camels in Saudi Arabia: proinflammatory cytokines, acute phase proteins and oxidative stress biomarkers. Pak Vet J 2014:34;484–488.
- #14. Meuten D. Parathyroid glands and calcium and phosphorus metabolic pathology. In: Thrall MA, et al., eds. Veterinary Hematology and Clinical Chemistry. 2nd ed. Ames, IA: WileyBlackwell, 2012:545–568.
- #20. Shantsila E, Lip GY. Systemic inflammation as a driver of vascular calcification: a proof of concept. J Intern Med 2009;266:453–456.
I am also providing a link to the excellent 1978 article by Dr. Claus Buergelt and colleagues on the pathology of bovine paratuberculosis which mentions aortic calcification.
______________
Comment: This report is a perfect example of how an inquisitive veterinary practitioner requested a necropsy on a cow with Johne's disease, how veterinary pathologists documented some novel pathology, how the practitioner reached out via the internet to learn more, and how personal and internet connections helped everyone reading this post to know more about the pathobiology of Johne's disease. In many ways it mirrors the how the first report of Johne's disease came to be.
______________
And.....for the keenly interested, here are some additional related references with links. This is not an exhaustive list. Sorry if I missed your favorite publication:
- US Sorge et al. Cow-level association between serum 25-hydroxyvitamin D concentration and Mycobacterium avium subspecies paratuberculosis antibody seropositivity: A pilot study. Journal of Dairy Science. 96(2):1030-1037, 2013.
- JR Stabel et al. Dietary calcium modulates Mycobacterium paratuberculosis infection in beige mice. Veterinary Immunology and Immunopathology, 66(3–4): 377-390, 1998.
- RW Jayalath et al. Aortic calcification. European Journal of Vascular and Endovascular Surgery. 30(5):476-488, 2005.
- N Niederhoffer et al. Aortic calcification produced by vitamin D3 plus nicotine. Journal of Vascular Research. 34(5):386-398. 1997.
« Previous 1 … 10 11 12 13 14 … 18 Next »