JOHNE'S IN CAPTIVE DEER
2019-09-17 16:38:18Research Report
 Dr. Mitch Palmer and colleagues from the USDA-ARS National Animal Disease Center in Ames, IA, USA. just published an article describing diagnostic testing patterns for paratuberculosis in a captive white-tailed deer farm in the U.S. The publication titled “Characteristics of subclinical Mycobacterium avium ssp. paratuberculosis infection in a captive white-tailed deer herd“ appears in the Journal of Veterinary Diagnostic Investigations; first published online September 11, 2019.
Dr. Mitch Palmer and colleagues from the USDA-ARS National Animal Disease Center in Ames, IA, USA. just published an article describing diagnostic testing patterns for paratuberculosis in a captive white-tailed deer farm in the U.S. The publication titled “Characteristics of subclinical Mycobacterium avium ssp. paratuberculosis infection in a captive white-tailed deer herd“ appears in the Journal of Veterinary Diagnostic Investigations; first published online September 11, 2019.
Abstract
Paratuberculosis (Johne’s disease) is caused by Mycobacterium avium ssp. paratuberculosis (MAP), and affects both domestic and wild ruminants, including cattle, goats, sheep, and deer. In cattle, most infections occur during calfhood followed by a prolonged incubation period of 1–2 y or more before cows shed culturable numbers of MAP bacilli in their feces. As disease progresses, infected animals develop protein-losing enteropathy, intractable diarrhea, and weight loss. In a cohort of 32 clinically normal deer from a herd with a history of periodic clinical paratuberculosis, we found that subclinical infection was characterized by high rates of infection, common involvement of mesenteric lymph nodes, minimal lesion formation, few intralesional acid-fast bacilli, and low-level fecal shedding of MAP. The characteristics of subclinical paratuberculosis in white-tailed deer resemble those of cattle and red deer, although microscopic lesions were less common in subclinical deer than reported for subclinical cattle, and we did not see necrotizing granulomas as described in subclinical red deer and elk.
Comment: This is another important contribution that highlights how different animal species handle MAP infections differently and that a prolonged period where animals are infectious (shedding MAP in feces) while appearing clinically normal fosters continued spread of the infection within and among animal herds. Unfortunately this article is not open access.
THE JOHNE’S VACCINE IN THE U.S. WILL SOON BE GONE
2019-09-10 16:01:42Excerpt from Hoard’s Dairyman September 10, 2019 issue, “Veterinary Column” (page 544).
Although there are many components to Johne’s control programs on dairy farms, the use of the Johne’s vaccine became important and relied upon for some producers. As such, the relatively recent news that the single manufacturer in the U.S. with federal licensure for vaccine production was no longer producing new vaccine came as a blow.
At this point in time there does not appear to be a replacement product. Similarly, none of the newer technology vaccines coming to market will have obtained federal approval by the time the existing stocks run out.
Veterinarians and producers on farms affected by the loss of vaccination may need to refocus their collective efforts on newborn calf and maternity pen management, manure handling and removal, and possibly some environmental testing and tough culling decisions for high shedding animals.
More background:
The U.S. Johne’s vaccine is called Mycopar™ and was sold by Boehringer-Ingelheim. Multiple academics report progress toward a more effective vaccine for Johne’s disease.
This links you to a 2011 review article about paratuberculosis and the role of vaccination for control.
This links you to a 2016 review article about Johne’s (paratuberculosis) vaccines.
The Guidair® and Silirum® vaccines (both sold by Zoetis, a sponsor of this website) are used outside the U.S. for protection of sheep and goats.
Comment: Commercialization of new vaccines for Johne’s disease is a high-risk high-reward challenge. Many companies are reluctant to take on this challenge because of the high cost and long time required to prove vaccine efficacy in each target animal species. However, given the significant and rising prevalence of MAP infections in multiple animal species and the likelihood that MAP is a food-borne zoonotic infection with worldwide distribution, there is huge economic potential for sale a truly safe and effective vaccine. Most producers would rather "control Johne's disease via syringe" rather than do the necessary herd management changes and diagnostic testing.
CONTRASTING TWO WASTING DISEASES
2019-09-04 20:15:54![]()
Chronic Wasting Disease (CWD) is in the news as experts raise concerns about its unchecked spread. Read here the Opinion/Hypothesis article in the July/August issue of mBio titled: Chronic Wasting Disease in Cervids: Implications for Prion Transmission to Humans and Other Animal Species. The authors of the article conclude: Available data indicate that the incidence of CWD in cervids is increasing and that the potential exists for transmission to humans and subsequent human disease. Given the long incubation period of prion-associated conditions, improving public health measures now to prevent human exposure to CWD prions and to further understand the potential risk to humans may reduce the likelihood of a BSE-like event in the years to come.
Today's Johnes.org news item contrasts CWD with a more common chronic wasting disease known as Johne’s disease (JD). I do this with the intention of questioning the relative importance of CWD and JD to society and urging science-based decisions on animal disease control investments. The evidence in the table below and the references that follow speak for themselves.
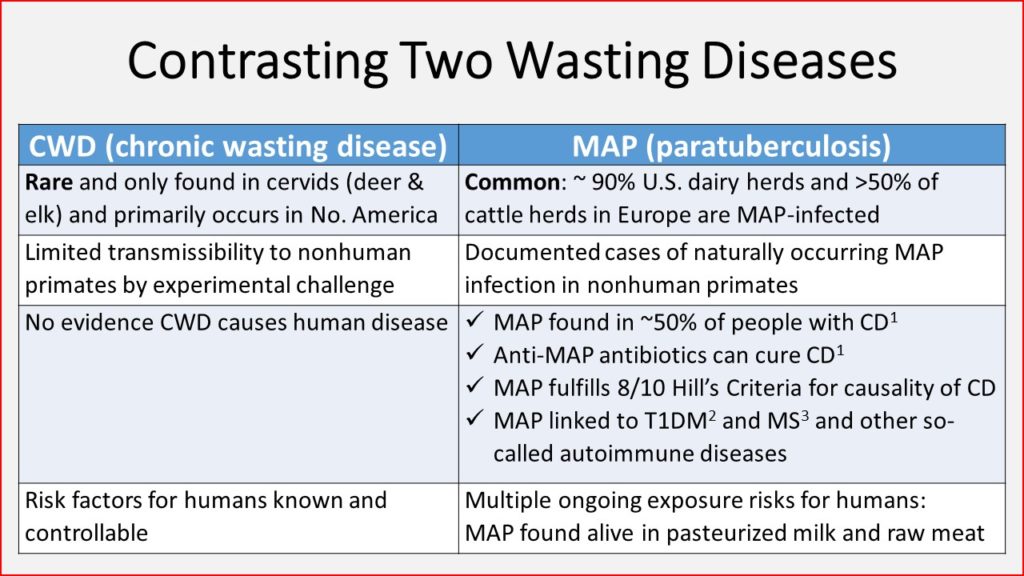
Footnotes:
- 1 Crohn’s disease affects more than 1 in 800 people in North America, and while the incidence has plateaued in more industrialized countries, since 1990 the incidence has been rising in newly industrialized countries in Africa, Asia, and South America, including Brazil.
- 2 Type 1 Diabetes Mellitus affects roughly 1 in 550 youth (<20 years old) in the U.S. and Canada, and the incidence is rising.
- 3 Multiple Sclerosis affects roughly 1 in 700 in the U.S., and the incidence is rising.
References and recommended reading:
- MAP as a zoonosis on this site: https://johnes.org/general-information/zoonotic-potential/
- Race, B. et al. 2014. Chronic wasting disease in nonhuman primates. Emerg Infect Dis 20(5): 833-837.
- Lombard, JE, et al. 2013. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Preventive Veterinary Medicine. 108:234-238.
- Nielsen, SS, and Toft, N. 2009. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev Vet Med 88:1-14.
- Ellingson, JLE, et al. 2005. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J Food Protection 68(5):966-972.
- Chiodini, R. et al. 2012. Crohn’s disease and the mycobacterioses: A quarter century later. Causation or simple association? Critical Reviews in Microbiology 38(1):52-93.
- Ng, SC. 2017. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 390:2769-2778.
- Waddell, LA, et al. 2015. Review Article: The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: a systematic review and meta-analyses of the evidence. Epidemiol Infect 143:3135-3157.
- National Advisory Committee on Microbiological Criteria for Foods. 2010. Review: Assessment of Food as a Source of Exposure to Mycobacterium avium subspecies paratuberculosis (MAP). J Food Protect 73(7):1357-1397.
- Sechi, L. et al. 2008. Humoral immune responses of Type 1 Diabetes patients to Mycobacterium avium subsp. paratuberculosis lend support to the infectious trigger hypothesis. Clinical and Vaccine Immunology Feb:320-326.
- SEARCH for Diabetes in Youth. 2006. The Burden of Diabetes Mellitus among US youth: Prevalence estimates. Pediatrics 118(4).
- Dilokthornsakul, P. et al. 2016. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 86(11).
- Cossu, D. et al. 2011. Association of Mycobacterium avium subsp. paratuberculosis with Multiple Sclerosis in Sardinian patients. PLoS One 6(4):e18482.
15TH INTERNATIONAL COLLOQUIUM ON PARATUBERCULOSIS
2019-09-02 14:45:36 Abstract submission for the 15th International Colloquium on Paratuberculosis to be held in Dublin, Ireland on June 14th to 18th has opened at https://www.icpdublin.com/abstract and will remain open until December 1st. Hope that you will submit an abstract! Looking forward to a great conference next Summer.
Abstract submission for the 15th International Colloquium on Paratuberculosis to be held in Dublin, Ireland on June 14th to 18th has opened at https://www.icpdublin.com/abstract and will remain open until December 1st. Hope that you will submit an abstract! Looking forward to a great conference next Summer.
Complete information about the upcoming 15-ICP can be found here.
MAP IN MILK FOR CALVES
2019-08-23 14:53:50 Dr. Pamela Steur published a study regarding MAP transmission on Chilean dairy farms as part of her doctoral dissertation in the laboratory of Dr. Miguel Salgado, Instituto de Medicina Preventiva Veterinaria, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile. Her article appears in the journal Tropical Animal Health and Production.
Dr. Pamela Steur published a study regarding MAP transmission on Chilean dairy farms as part of her doctoral dissertation in the laboratory of Dr. Miguel Salgado, Instituto de Medicina Preventiva Veterinaria, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile. Her article appears in the journal Tropical Animal Health and Production.
This interesting study found an association between the seroprevalence of MAP infections in dairy herds based on ELISA (IDEXX) and the number of MAP in milk intended for feeding to calves. MAP detection in milk was enhanced by use of peptide-mediated magnetic separation (PMS) technology as developed and refined in the laboratory of Dr. Irene Grant, Queens University Belfast. Up to 1 million MAP per milliliter of milk were detected. This figure from their publication illustrates the association of herd seroprevalence and level of MAP in milk intended for feeding to calves.
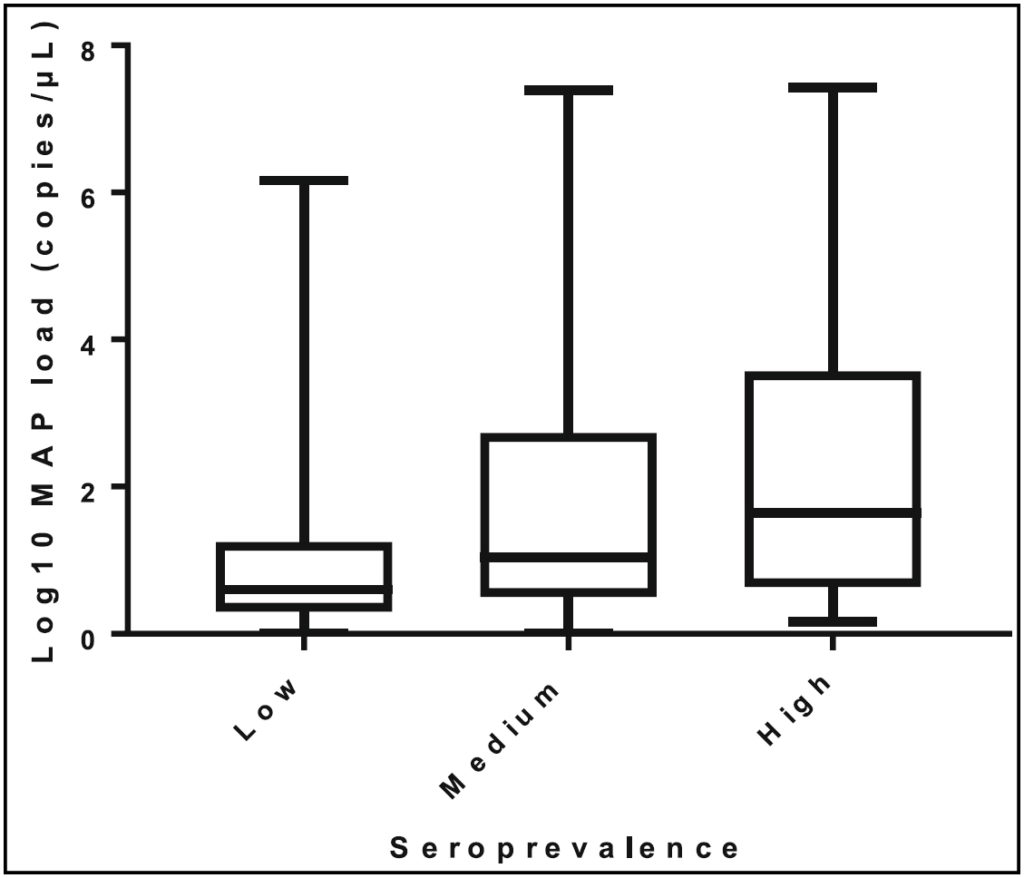
Comments: This study highlights how a MAP infection epidemic gains momentum as the number of cows shedding MAP steadily rises and the rate of contamination of milk being fed to calves increases in parallel. Milk is perhaps the most efficient vehicle for transmission of MAP to the most susceptible animals in dairy herds, calves. Some of the MAP in milk is directly excreted from the udder but the majority probably come with fecal contamination of the milk. While dairy producers in some countries can afford to use on-farm pasteurizers or purchase calf milk replacer (pasteurized powdered milk) as a means of protecting calves from MAP, this is not always an option. Moreover, when bulk feeding of milk to calves is practiced (instead of milk from one cow to one calf) the infection rate accelerates quickly. Pictured here is an example of a “calfeteria” in use on a Chilean dairy farm.

JOHNE’S DISEASE: CHEAP TO BUY, COSTLY TO LIVE WITH
2019-08-16 15:32:13 This is the title of an August 15 blog posting by the Beef Cattle Research Council (BCRC) in Canada. The author uses Johne’s disease as an example about the broader issues of farm biosecurity.
This is the title of an August 15 blog posting by the Beef Cattle Research Council (BCRC) in Canada. The author uses Johne’s disease as an example about the broader issues of farm biosecurity.
Here is an excerpt:
A well-run herd can introduce and spread Johne’s disease just by buying the wrong animal. Johne’s cannot be prevented by vaccination or effectively treated by antibiotics, and accurately identifying and culling infected animals is very difficult before they get visibly ill. Johne’s disease also can’t be prevented by high herd health, nutritional, grazing, or genetic management. Many beef producers may think they have closed herds, but realistically speaking hardly anyone does. Appropriate biosecurity is the best thing producers can do to help keep Johne’s from entering their herds.
The article goes on to describe the prevalence of Johne’s disease in Canadian beef cattle herds and much more. So, check it out!
ANOTHER JD CONTROL SUCCESS STORY!
2019-08-13 16:14:17 Management decisions to protect calves from infection such as separation of infected cows at calving and discard of calves, milk and colostrum from MAP positive cows, or pasteurization of their milk, are uncommon in seasonal, pastoral New Zealand (NZ) dairy farming. Pasture management is greatly complicated by any increase in the number of groups of grazing cows. The NZ Animal Compounds and Veterinary Medicines act (1987) prohibits the sale of milk for human consumption when that milk is contaminated with drug residues. Consequently, calves are commonly fed on milk from sick cows, those undergoing antimicrobial treatment or excluded from the main herd for other reasons.
Management decisions to protect calves from infection such as separation of infected cows at calving and discard of calves, milk and colostrum from MAP positive cows, or pasteurization of their milk, are uncommon in seasonal, pastoral New Zealand (NZ) dairy farming. Pasture management is greatly complicated by any increase in the number of groups of grazing cows. The NZ Animal Compounds and Veterinary Medicines act (1987) prohibits the sale of milk for human consumption when that milk is contaminated with drug residues. Consequently, calves are commonly fed on milk from sick cows, those undergoing antimicrobial treatment or excluded from the main herd for other reasons.
Therefore, in NZ, there has been relatively little engagement from dairy farmers in the control of JD unless they have experienced a high clinical prevalence and there is evidence of increasing prevalence of JD especially in the South Island of the country.
Andrew Bates et al., Vetlife Centre for Dairy Excellence, Geraldine, NZ reported in BMC Veterinary Research (Open Access) the results of a single herd study where a high prevalence of clinical JD and MAP infection was reduced over a 4 year period using an annual test and cull approach. The strategy was based on a herd testing protocol using an initial herd screening using serological ELISA coupled with a quantitative fecal PCR (fPCR) test to confirm the status of ELISA-positive animals.
Over the 4 year period a total of 4,358 blood samples were submitted from 2,211 cows and of these 683 were submitted for fPCR. Culling decisions followed a decision tree approach shown below. To aid the removal of animals shedding large numbers of MAP, a priority was made to remove all animals with a high fPCR status, followed by those that were had high ELISA results.
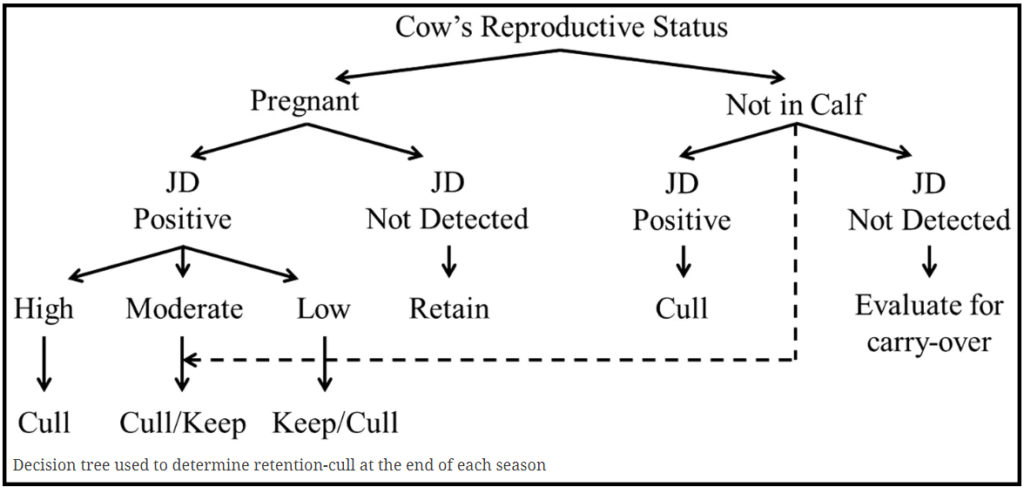
For all age groups considered the apparent seroprevalence of cows testing positive decreased from 26% in 2013–2014, to 2.3% in 2016–2017. The reported proportion of calved cows culled annually from suspected clinical Johne’s disease fell from 5% in the year preceding the control program to 0.4% in the final year of the study.
On this farm, reduction in the prevalence of infection was achieved by reducing the infectious pressure through targeted culling of heavily shedding animals together with limited measures to protect calves at pasture from exposure to Mycobacterium avium subsp. paratuberculosis (MAP).
This study demonstrates that - with a combination of pre-calving diagnostic testing to identify and remove animals that are the major source of infectious spread, coupled with simple management changes to physically separate replacement calves from MAP infected adult cattle - effective reduction in the prevalence of JD is possible for NZ dairy farmers.
Comment: This important study validates that removing the most infectious animals from a herd significantly decreases the prevalence of the infection. However, it ignores the economic utility of the program, i.e. the cost-benefit of this approach. If the ELISA was valued at US$5.00 and the fPCR at US$30 (typical costs in U.S. labs), then the cost of this control program, in laboratory testing costs alone, was $42,280. Most herd owners would question the return on investment without receiving some compensation from the processor buying his/her milk or from a governmental agency concerned with food safety.
BMC EXPANDS DISCUSSION OF INTERNATIONAL SURVEY
2019-08-09 17:01:21
The study recently published in BMC Veterinary Research, which surveyed 48 countries around the world, highlighted the crucial need to secure funding and international support for implementing long-term veterinary control programs against paratuberculosis. BMC publishers asked Emeritus Professor Richard Whittington, who led and formed the network of international experts, more about the disease, how the survey was carried out and the implications of the survey findings. The blog title is: Q&A on Paratuberculosis in livestock: insights to a neglected disease and experts’ recommendations.
If you missed it, this takes you to the original survey publication.
LOW MAP INFECTION PREVALENCE IN COLUMBIA
2019-07-25 17:09:28Research Article
 Nathalia M. Correa-Valencia and colleagues reported on the herd-level prevalence of paratuberculosis in Northern Antioquia, Columbia. Their study, titled: Prevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds in Northern Antioquia (Colombia) and associated risk factors using environmental sampling, appears in the latest issue of Preventive Veterinary Medicine. In comparison to other countries, the prevalence of paratuberculosis in dairy herds in this region of Columbia is low. This finding should increase efforts to protect the as yet non-infected herds.
Nathalia M. Correa-Valencia and colleagues reported on the herd-level prevalence of paratuberculosis in Northern Antioquia, Columbia. Their study, titled: Prevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds in Northern Antioquia (Colombia) and associated risk factors using environmental sampling, appears in the latest issue of Preventive Veterinary Medicine. In comparison to other countries, the prevalence of paratuberculosis in dairy herds in this region of Columbia is low. This finding should increase efforts to protect the as yet non-infected herds.
Abstract
This cross-sectional study aimed to determine Mycobacterium avium subsp. paratuberculosis (MAP) herd-level prevalence using a quantitative real-time PCR method (qPCR), performed on environmental samples. Secondly, the study aimed to explore herd-level risk factors associated with the presence of MAP in dairy herds with in-paddock milking facilities of the Northern region of the Province of Antioquia (Colombia). Study herds (n = 292) located in 61 different districts from six municipalities were randomly selected amongst 7,794 dairies registered in the foot-and-mouth disease vaccination records from 2015. The sampling strategy considered a proportional allocation, both at municipality and district level. Participant herds were visited once between June and October 2016 to collect one composite environmental sample and to complete a risk assessment questionnaire. Each composite environmental sample contained material from six different sites of concentration of adult cattle and/or high traffic areas (e.g. areas surrounding waterers and feeders, areas surrounding the current mobile milking-unit places). Identification of MAP was achieved using a duplex qPCR (Bactotype MAP PCR Kit®, Qiagen). A herd was considered as MAP infected if the environmental sample was positive in the qPCR. Information about the general characteristics of the herd, management practices, and knowledge about the disease was collected using the risk-assessment questionnaire. The information on risk factors was analyzed using a multivariable logistic regression model. The apparent herd-level prevalence was 4.1% (12/292; 95% CI: 1.8-6.4). Herds with a history of mixed farming of cattle with other ruminants had higher odds of being MAP infected than herds without (OR = 3.9; 95% CI: 1.2-13.2). Our study demonstrates the MAP prevalence in dairy herds from Antioquia, Colombia and the possible relationship between MAP environmental positivity with the history of mixed farming of cattle with other susceptible ruminants.
This map from Wikipedia shows the location where the study was done.
Comment: Lombard et al. reported results from a survey of U.S. dairy herds using similar surveillance methods (Preventive Veterinary Medicine 108:234-238). In that NAHMS Dairy 2007 study, the apparent herd-level prevalence of MAP was 70.4% (369/524 had ≥1 culture-positive composite fecal samples out of 6 tested). Adjusting for the estimated herd sensitivity and herd specificity of the test method, the estimated TRUE herd-level prevalence of paratuberculosis in U.S. dairy herds was 91.0% (95% probability interval, 81.5 to 99.3%).
ACCURACY OF A MILK ELISA IN GOATS
2019-07-08 18:19:41Research Article – Open Access
 Luttikholt et al. from the Department of Small Ruminant Health, GD Animal Health, The Netherlands, reported on the accuracy of an ELISA (IDEXX) used on milk samples from goats in both MAP-vaccinated and non-vaccinated herds. The cut-off for a positive test was based on ROC analysis and set at 25 S/P%. The cut-off recommended by the kit manufacturer for bovine milk samples is ≥30 S/P%. This article appears in the July 2019 issue of Veterinary Sciences.
Luttikholt et al. from the Department of Small Ruminant Health, GD Animal Health, The Netherlands, reported on the accuracy of an ELISA (IDEXX) used on milk samples from goats in both MAP-vaccinated and non-vaccinated herds. The cut-off for a positive test was based on ROC analysis and set at 25 S/P%. The cut-off recommended by the kit manufacturer for bovine milk samples is ≥30 S/P%. This article appears in the July 2019 issue of Veterinary Sciences.
Abstract
The aims of our study were to calculate the most appropriate cut-off value for milk samples in a serum-validated Mycobacterium avium subsp. paratuberculosis (MAP) ELISA and to analyze MAP ELISA responses in milk samples from vaccinated and non-vaccinated dairy goats in the Netherlands. Analyzed herds were representative for location and herd size of dairy goat herds in the Netherlands. A significantly higher proportion of the analyzed 49 herds were organic as compared with the total Dutch dairy goat population. First, the MAP ELISA was optimized using 992 paired serum and milk samples. At a cut-off of 25 S/P%, the relative sensitivity (Se) was 58.4% (n = 992, 95% CI: 48.8%−67.6%) and relative specificity (Sp) was 98.5% (n = 992, 95% CI: 97.5%−99.2%), as compared to serum ELISA results. The percentage of positively tested herds was 78.2% (n = 49, 95% CI: 63.4%−88.1%). The percentage of positive milk samples per herd (n = 22) was on average 4.6% (median, min, and max of 4.7%, 0.0%, and 10.7%, respectively). Average age of ELISA-positive (3.2 years) and -negative goats (3.2 years) was not different. Significantly more vaccinated goats tested positive (6.7%) as compared with non-vaccinated goats (1.1%). This study shows that a high number of vaccinated and non-vaccinated commercial dairy goat herds in the Netherlands have MAP-ELISA-positive goats.
Comments
Clearly, MAP infections are common in Dutch dairy goat herds (78.1% had 1 or more milk ELISA-positive animals) which somewhat higher than MAP infection prevalence estimates among goat herds in other countries (see the discussion section of the paper and previous news items on this site). MAP vaccination increased the rate of animals testing positive by the milk ELISA but, as the authors acknowledge, this could be because vaccinating herds have a higher infection rate, i.e. the reason they started vaccinating for paratuberculosis.
This study compared an ELISA on milk samples to the same ELISA applied to serum samples as the reference test. Normally the reference test should be a more sensitive assay such as fecal culture or fecal PCR – sometimes called “gold standard” assays. By using the serum ELISA as a reference test the reported sensitivity of the milk ELISA is somewhat inflated because the serum ELISA sensitivity in goats when compared to fecal culture has been reported as 74.3% using fecal culture as the reference test by traditional sensitivity estimate methods (Salgado et al., 2007) and 63% using Bayesian methods and including fecal culture results (Kostoulas et al, 2006). Thus, if we assume that the serum ELISA reference test in this study detected 68.6% of all truly MAP-infected animals (based on the mean sensitivity reported by two published studies I cited) and the present study found that only 58.4% of the serum ELISA-positive animals were also milk ELISA-positive, one could estimate that true sensitivity of the milk ELISA in goats for detecting MAP infected animals shedding MAP in feces was at most 40% (58.4% x 68.6%). Had the authors used the cut-off recommended for this ELISA when bovine milk samples, the sensitivity estimate would be lower still.
Use of low sensitivity tests works well for herds, regions or countries wanting to demonstrate that MAP is not a big problem and/or to lessen consumer concerns. However, it can misrepresent the true magnitude of the paratuberculosis problem and lead herd owners to under-estimate the significance of MAP infections to the health of their herd.
« Previous 1 … 12 13 14 15 16 … 18 Next »