POTENTIAL COST OF MAP IN MILK
2020-11-29 15:44:06L. Chiu and colleagues from Cornell University and the University of Illinois published a research article exploring the societal costs of MAP in the milk supply and a link of MAP to Crohn’s disease. Their work was published in the International Journal of Food System Dynamics [Open Access].
ABSTRACT
Welfare costs of a potential food shock were estimated by disseminating information to milk drinkers on the prevalence of Mycobacterium avium sub. paratuberculosis (MAP) in the U.S. milk supply, its potential linkage to Crohn’s disease in humans, and subsequent government intervention to minimize MAP in the milk supply. We found that 19.6% of milk consumers exposed to MAP information would stop milk consumption at current market prices, and that only 5% of those would return to their original milk consumption levels after the government intervention. Societal costs of the food shock after the intervention were estimated at $18.2 billion.
RELATED STUDY
A similar study by H. Groenendaal and Zagmutt was published in the Journal of Dairy Science in 2008. This article, not cited by Chiu, explored three scenarios developed based on the effectiveness of possible risk-mitigation strategies. As reported in their publication, “in the first scenario, it was assumed that an effective strategy exists; therefore, a negligible demand decrease in the consumption of dairy products was expected. In the second scenario, it was assumed that new risk mitigation would need to be implemented to minimize the health hazard for humans. In this case, a small milk demand decrease was expected, but larger demand decreases were also possible. The third scenario assumed that no fully effective risk mitigation was available, and this resulted in a considerable demand decrease and a potential reduction in milk supply as a result of regulatory measures. A milk demand reduction of 1 or 5% resulted in a reduction in consumer surplus of $600 million and $2.9 billion, and a reduction in dairy farm income of $270 million and $1.3 billion, respectively. A decrease in milk supply would cause a slight increase in total losses, but would cause the greatest losses to test-positive dairy farms. Given the current scientific knowledge about MAP and CD, we conclude that if a link were established, it is most likely that the first or second scenario would occur. Thus, consumer response and economic consequences to the discovery of such a link are expected to be limited, but could be large if the consumer's perception of risk is large or if risk-mitigation strategies were ineffective.”
COMMENT
Prevention pays! Dairy producers and processors should work to limit the potential impact of MAP on consumer acceptance of dairy products. The necessary diagnostic tools and knowledge on how to control MAP infections in dairy cattle are available. Some countries have implemented national control programs to help avert negative consumer reactions should medial science accept that MAP is a zoonotic pathogen. Actions to control MAP in dairy cattle can protect consumer confidence while also improving farm profitability and animal health and welfare.
JD CAUSES FALSE-POSITIVE TB TESTS IN CATTLE
2020-11-23 14:05:38 Mariana Assunção de Souza and 6 other Brazilian colleagues reported on the occurrence of Johne’s disease (paratuberculosis) in cattle that were necropsied based on a positive comparative cervical skin test for bovine tuberculosis (bTB). Their research article appears in the current issue of Acta Scientiae Veterinariae.
Mariana Assunção de Souza and 6 other Brazilian colleagues reported on the occurrence of Johne’s disease (paratuberculosis) in cattle that were necropsied based on a positive comparative cervical skin test for bovine tuberculosis (bTB). Their research article appears in the current issue of Acta Scientiae Veterinariae.
Abstract
Background: Bovine tuberculosis control programs are based on a standard diagnostic method, the intradermal test with purified protein derivatives, which is used to identify and eliminate diseased animals. Currently none of the tests available allow complete differentiation between infected and uninfected animals. The main limitations of the tests available are related to diagnostic sensitivity and specificity, which results in false-positive reactions due to the existence of cross infections, and also false-negative, inherent to the state of energy of some animals. The aim of this work was to study the intercurrence of paratuberculosis in tuberculosis reactive cattle by the comparative cervical test.
Materials, Methods & Results: Three hundred and thirty-four cattle were evaluated using the comparative cervical test (CCT) and serology for tuberculosis (TB) and paratuberculosis (PTB) ELISA IDEXX®. All of the animals testing positive by CCT were euthanized and necropsied. Fragments of lymph node, lung and intestine were collected and analyzed using histopathological techniques, with staining by Hematoxylin-Eosin (HE). Samples of lung and lymph nodes (retropharyngeal, submandibular, cervical and mediastinal) of the animals testing positive by CCT were evaluated using qPRC for M. bovis, and intestinal and mesenteric lymph nodes using PCR for PTB. Of the 334 cattle evaluated using the comparative cervical test, 16 were considered positive. No lesions suggestive of tuberculosis were found in the macroscopic inspection of the carcasses. The most evident anatomical and pathological finding was a thickening of intestinal mucosa, found in 12 of the 16 cattle submitted to necropsy. No microscopic lesions suggestive of TB were identified nor was the presence of M. bovis detected by qPCR. The main histopathological findings were observed in the small intestine and mesenteric lymph nodes and identified as enteritis, lymphangitis, lymphangiectasia and granulomatous lymphadenitis. In the intestine the changes are characterized by dilated and inflamed lymphatic vessels and intense inflammatory infiltrate on the mucosa and submucosa. Of the 334 serum samples evaluated, the M. bovis ELISA Antibody Test (IDEXX®) identified 17 positive animals. All the cattle considered positive by M. bovis ELISA were considered negative by CCT. In the samples from nine animals (9/16), DNA from M. avium subsp. paratuberculosis (MAP) was identified and in twelve carcasses (12/16) lesions characteristic of PTB were found, which were subsequently confirmed by histopathological techniques. In another nine animals of the herd anti-MAP antibodies were detected. None of those that tested positive by PTB ELISA were reactive by CCT.
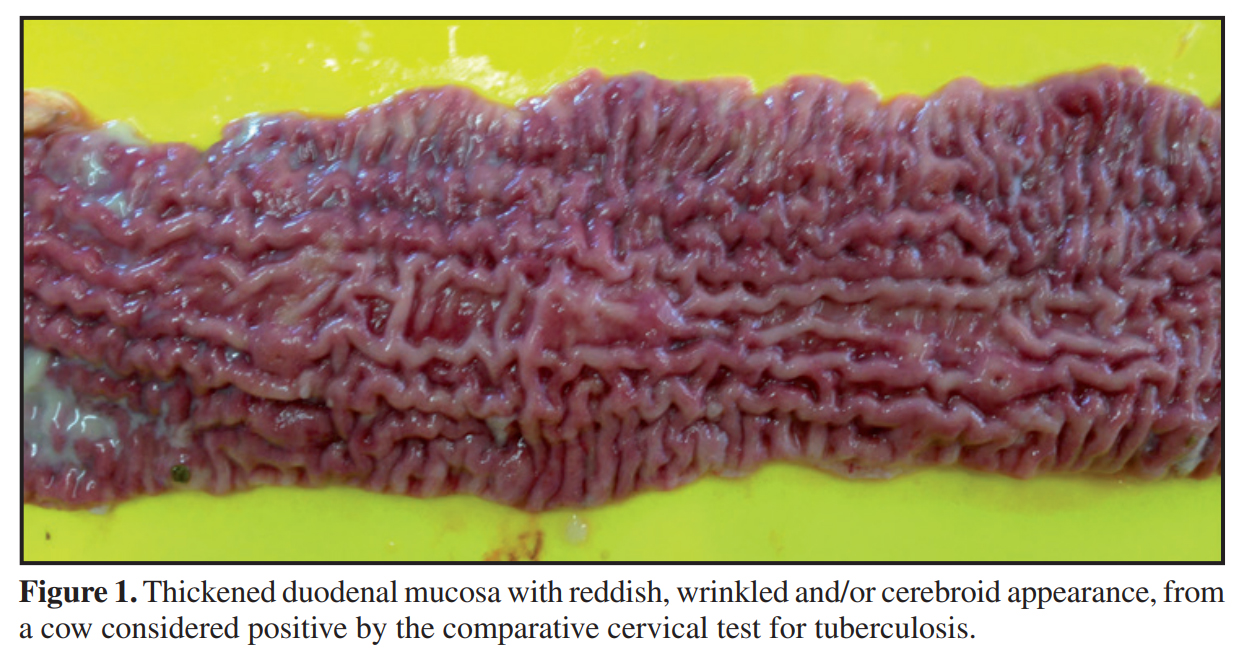
Discussion: Animals considered positive by TB ELISA that were not positive in the intradermal test does not mischaracterize the clinical picture of the disease. Considering the inverse relationship between cell-mediated and humoral responses to M. bovis, the intradermal test and the serological tests are designed to measure different immunological responses, which develop during different stages of infection. The progress of the cellular immunological response to humoral immunity occurs in the most advanced stages of tuberculosis. Of the 16 cattle considered positive by CCT, 12 animals presented macroscopic and histological lesions suggestive of PTB and DNA from MAP was detected in nine. Although it is the official test for the control of TB in different countries, the intradermal test with PPD has presented limitations, primarily related to specificity. M. avium subsp. Paratuberculosis is considered the main cause of false positive reactions in the intradermal test. The PPD bacterial extract is a complex mixture of proteins, lipids, sugars and nucleic acids, and many of these components are also shared by numerous species of mycobacteria (tuberculous or not).

The Brazilian study illustrates the interaction of MAP and M. bovis, the cause of bovine tuberculosis (bTB), as a confounder when trying to use standard immune-diagnostic tests for bTB. Other authors have found similar results. Out October 30 news posting on this site discussed the need to wait at least 60 days after skin testing cattle for bTB before using serological tests (ELISA) for Johne’s disease (JD) in order to avoid false-positive JD blood tests.
In a different but related context, other authors have likewise noted this interaction between mycobacterial diseases such as TB, leprosy and those caused by non-tuberculosis mycobacteria (NTM). The most common NTM infections are due to bacteria in the Mycobacterium Avium Complex (MAC) which includes the cause of JD, i.e. MAP.
In a letter published in the journal Inflammatory Bowel Diseases in 2008, Dr. Marcel Behr from McGill University Health Centre, Montreal, Canada highlighted instances where one mycobacterial infection interferes with another. One example he cited is that TB and leprosy (caused by Mycobacterium leprae) behaved as antagonistic epidemics – one infection blocks the other. When human TB is controlled, as it has in most developed countries, and when BCG vaccination of humans stops, other mycobacterial infections rise in prevalence. The theory is that the absence of one mycobacterial infection, e.g. TB, causes an “immunological void”, i.e. an increased susceptibility of humans to other mycobacteria that now find more susceptible, immunologically naïve, hosts. Note: BCG is a live non-virulent vaccine used to control TB in humans in countries where the disease is endemic. This vaccine was originally derived from M. bovis.
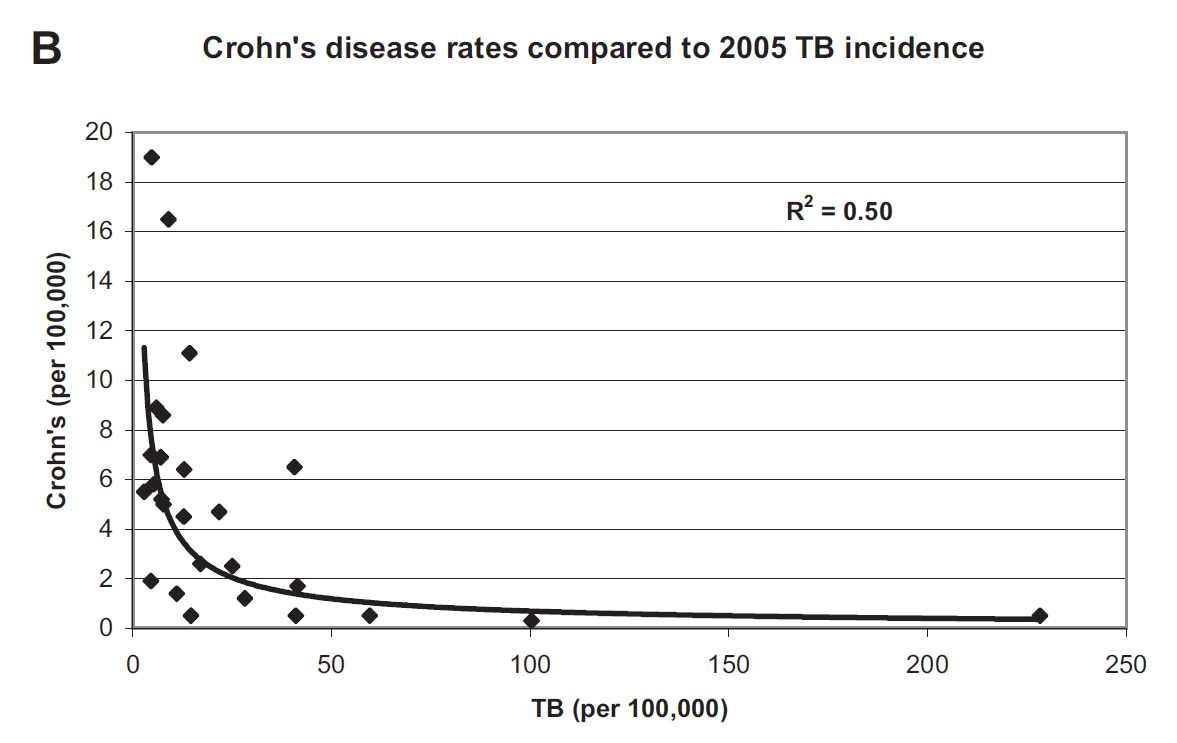
Because MAP is strongly associated with Crohn’s disease, Dr. Behr noted that Crohn’s disease (CD) was more common in countries where TB was less common. Below is the graphic he published illustrating inverse association between TB incidence and CD incidence. This is yet another piece of evidence for mycobacterial, e.g. MAP, involvement in CD.
Related to this is the review article by Dr. Dow (15 pages with 173 references) published in February 2020 on the influence of BCG vaccination on a variety of autoimmune diseases. In that article he states: "MAP has been associated with an increasingly long list of inflammatory/autoimmune diseases: Crohn's disease, sarcoidosis, Blau syndrome, Hashimoto’s thyroiditis, autoimmune diabetes (T1D), multiple sclerosis (MS), rheumatoid arthritis, lupus and Parkinson’s disease. Epidemiologic evidence points to BCG providing a “heterologous” protective effect on assorted autoimmune diseases; studies using BCG vaccination for T1D and MS have shown benefit in these diseases." His article proposes that the positive response to BCG in T1D and MS is due to a mitigating action of BCG upon MAP infections.
For more on the potential of MAP to cause Crohn’s disease visit this page of our site. Or listen to a 10-minute presentation titled: MAP is a zoonotic pathogen.
15-ICP POSTPONED AGAIN
2020-11-20 16:09:49
In March 2020, in response to the global pandemic brought about by the COVID-19 virus the Committee of the 15th International Association for Paratuberculosis Colloquium 2020 made the hard decision to postpone ICP 2020 until 2021. The event was originally scheduled for 14th -18th June, 2020 in Dublin Castle. The 15th International Colloquium for Paratuberculosis was then re-scheduled for the 6th – 9th April 2021.
Last month the Local Organizing Committee (LOC) was faced with the decision of having to postpone again or to have a virtual conference next April. It was decided by the LOC to go for a live conference in June 2022. The committee was delighted that the organizers of the 16th ICP in Jaipur, India agreed to this and have postponed their Colloquium until 2024.
The ICP Committee invites you to the 15th IAP Colloquium in Dublin, Ireland in June 2022. Delegates attending the conference can be assured of a productive and memorable colloquium, discover Irish heritage, culture and music and of course, experience the world-renowned hospitality of Ireland.
OJD REVISITED – AUSTRALIA
2020-11-13 17:54:34
Dr. Peter Windsor and Dr. Richard Whittington published a review article on ovine Johne’s disease (OJD) control in Australia. This excellent review article appears in the journal Animals and is Open Access. It nicely reviews the pathogenesis and control of paratuberculosis in sheep, but the most interesting part of the article addresses the top 10 subjects of misinformation about OJD in Australia.
Abstract
OJD is no longer the serious animal health issue that it was for many Australian rural communities a decade and a half ago. Despite declining OJD prevalence as determined by abattoir surveillance, the disease continues to spread, with OJD extension programs required to continually address the misinformation promulgated by some disaffected producers as new areas have become affected. Improved regional and on-farm biosecurity, including the introduction of a risk-based trading system, may have contributed to improved attitudes to OJD control, although attitudinal differences between OJD endemic areas and where the disease is not well established remain. Declines in on-farm OJD prevalence are almost certainly attributable to the widespread uptake of vaccination programs, although encouraging the ongoing use of vaccination to prevent recrudescence and improved biosecurity when mortalities disappear, remains challenging. Vaccination has provided a robust strategy for managing OJD and contributed significantly to the health of Australian sheep and the lives of producers with affected properties. As vaccination offers a pathway to reduce the risk of MAP infection entering the human food chain from small ruminant products, it should be more widely adopted globally, accompanied by research efforts to improve efficacy and importantly, the safety of vaccination to both operators and livestock.
Comment: Not all countries, such as the U.S., have access to the vaccine for paratuberculosis that is used in Australia. For pictures of sheep before and after developing clinical Johne’s disease can be found here.
JD IN SCIMITAR ORYX
2020-11-06 17:13:38Claudio Pigoli and colleagues report on multiple cases of Johne’s disease in captive scimitar-horned oryx at a zoo in Italy. Their research article was published in the journal Animals and is Open Access. Genomic analysis shows that the MAP strains recovered from the Oryx are closely related to those isolated from cattle in Italy: evidence of ongoing spillover from domestic livestock to wildlife and animals in zoos.

Abstract
Paratuberculosis, a chronic disease caused by Mycobacterium avium subsp. paratuberculosis (MAP), in ten scimitar-horned oryxes (SHOs) hosted in an Italian zoological park and originating from a Slovakian flock, was documented by pathology, molecular, cultural, and serological testing. The infection origin in this threatened species was also investigated by genomic analyses. Following the death of six of the 10 SHOs, serial investigations of dead and alive animals were performed. Necropsy, carried out on five out of six animals, identified intestinal thickening and mesenteric lymphadenomegaly in one of the animals. Histopathology (5/6) revealed lepromatous (2/5) and tuberculoid (2/5) intestinal forms or lack of lesions (1/5). Ziehl-Neelsen and immunohistochemistry stains identified two multibacillary, two paucibacillary forms, and one negative case. MAP was identified by quantitative PCR (qPCR) in tissue samples in five out of five SHOs and was microbiologically isolated from two of the three animals whose fresh tissue samples were available. Fecal samples were collected in four of the six dead animals: all four resulted positive to qPCR and in MAP was isolated in three. ELISA identified MAP-specific antibodies in three of the five dead animals whose serum was available. qPCR identified MAP in the freshly deposited feces of two out of the four alive animals. From the feces of these two animals, MAP was microbiologically isolated in one case. All isolates were classified as MAP type C and profiled as INMV2 and MVS27 by molecular analysis. Genomic analysis of a field isolate revealed clustering with a European clade but was more similar to Italian than East European isolates. Our findings underline that paratuberculosis should always be considered in zoological parks in which endangered species are hosted. Infection can be subclinical, and multiple combined testing techniques may be necessary.
Author’s Conclusions
Our results underline the importance of considering paratuberculosis in zoological parks, where endangered species are often hosted. Paratuberculosis could represent a risk for the conservation of rare animals, and it is essential to include it in the panel of diagnostic tests to be performed on hosted animals. We also suggest testing dead animals, in which different diagnostic approaches are combined, with the final aim of fully elucidating the causes of death and defining their health status regarding paratuberculosis. WGA can help to trace the origin of infections, particularly in the case of moved animals. This study reports the first genome of an MAP strain isolated from SHOs and shows that the strain likely derived from the Italian cattle livestock, in which MAP is endemic.
For more on Johne’s disease in zoo animals check out this page of our website.
MILK ELISA & DAIRY CATTLE HERDS
2020-10-30 19:53:25Two Related Research Publications
Two excellent articles about use of the ELISA diagnostic test on milk samples (milk ELISA) appeared in the past few weeks. The first, by Ozsvari and colleagues uses the milk ELISA to evaluate the impact of paratuberculosis on milk production, fertility, and culling in large commercial dairy herds in Hungary. This article published in Frontiers in Veterinary Science is Open Access.
Abstract
Paratuberculosis (PTBC) is a chronic disease caused by Mycobacterium avium subsp. paratuberculosis (MAP), which is common in dairy herds worldwide, although the scale of its impact on herd productivity is unclear. The aim of our study was to determine the differences between MAP ELISA positive vs. negative cows in terms of milk production and quality, reproductive parameters, and culling. The data of five large dairy herds that participated in the voluntary PTBC testing program in Hungary were analyzed. Cows were tested by ELISA (IDEXX Paratuberculosis Screening Ab Test, IDEXX Laboratories, Inc., Westbrook, ME, USA) using milk samples collected during official performance testing. The outcome of the initial screening test involving all milking cows in the herds was used for the classification of the cows. The 305-day milk production, reproduction and culling data of 4,341 dairy cows, and their monthly performance testing results (n = 87,818) were analyzed. Multivariate linear and logistic models, and right censored tobit model were used for the statistical analysis. Test-day and 305-day milk production of ELISA positive cows decreased by 4.6 kg [95% CI: 3.5–5.6 kg, P < 0.0001 (−13.2%)] and 1,030 kg [95% CI: 708–1,352 kg, P < 0.0001 (−9.4%)], compared to their ELISA negative herdmates, respectively. Milk ELISA positive cows had 35.8% higher [95% CI: 17.9–56.4%, P < 0.0001] somatic cell count, on average. Test positive cows conceived 23.2 days later [95% CI: 9.2–37.3 days, P = 0.0012 (+16.5%)] and their calving interval was 33.8 days longer [95% CI: 13.2–54.4 days, P = 0.0013, (+9.7%)], compared to the negative cows, on average. Milk ELISA positive cows were less likely to conceive to first insemination (odds ratio: 0.49, 95% CI: 0.31–0.75, P = 0.0013), and required 0.42 more inseminations to conceive [95% CI: 0.07–0.77, P = 0.0192 (+13.7%)], on average. Milk ELISA positive cows were culled 160.5 days earlier after testing compared to their ELISA negative herdmates (95% CI: 117.5–203.5 days, P < 0.0001). Our results suggest that MAP ELISA positive cows experience decreased milk production, milk quality, fertility, and longevity, which supports the need to control the prevalence of PTBC in dairy herds.
______________
 The second publication is by Barden and colleagues from the U.K. describes the effect of skin testing for bovine tuberculosis (TB) on milk ELISA results. This article published in the journal Preventive Veterinary Medicine is available online only until November 29.
The second publication is by Barden and colleagues from the U.K. describes the effect of skin testing for bovine tuberculosis (TB) on milk ELISA results. This article published in the journal Preventive Veterinary Medicine is available online only until November 29.
Abstract
Background: In the UK, quarterly Johne’s disease milk antibody ELISAs (JD-mELISAs) are commonly used to classify animals which are likely to be infectious, termed “red cows”. “Red cows” are classified following two positive results from the previous four tests (e.g. + - - +). All cattle are also regularly screened for bovine tuberculosis using intradermal avian and bovine tuberculin, and it is advised to maintain a 60 day interval between a tuberculosis test and JD-mELISA. Aims: To evaluate the impact of bovine tuberculosis testing on JD-mELISAs, and to quantify the impact of test specificity and “red cow” classification test pattern on the probability of infection.
Methods: Four years of individual cow milk records with JD-mELISA results were collated from 735 dairy farms and matched to tuberculosis testing records. A two-level multivariable logistic regression model quantified the effect of tuberculosis testing on JD-mELISA result. The specificity and age-dependent sensitivity of a single JD-mELISA were estimated and used to calculate likelihood ratios following each test. Using Bayes’ theorem, the posterior probability of infection with Johne’s disease was calculated for different specificities, ages of cow, and patterns of test results.
Results: There were increased odds of a positive JD-mELISA if it was ≤30 days (OR: 2.1) or 31− 60 days (OR: 1.2) after a tuberculosis test, compared to >90 days. A larger avian skin reaction at the tuberculosis test was also associated with increased odds of a positive JD-mELISA. The proportion of cows which tested exclusively negative after their first positive JD-mELISA was higher if that JD-mELISA was ≤30 days after a tuberculosis test compared to >90 days. The posterior probability of infection reduced substantially when the test specificity was slightly reduced. In “red cows” classified following two consecutive positive tests, if the test specificity was reduced to 0.95, then the posterior probability of infection was only >95 % if the prior probability was >13 %. If the “red cow” classification was due to two non-consecutive positive tests (+ - - +), the posterior probability of infection was only >95 % if the prior probability was >43 %.
Conclusions: Testing for Johne’s disease within 60 days of a tuberculosis test is associated with a higher chance of a positive JD-mELISA and this may reflect a reduction in the ELISA specificity. Relatively small reductions in JD-mELISA specificity can markedly reduce the posterior probability of infection which also depends on the pattern of test results which classifies “red cows”. [Red cows are those with 2 consecutive positive milk ELISA tests.]
____________
Comment: The milk ELISA is the most affordable testing option for commercial dairy cattle herds. It primarily identifies cows in the more advanced stages of a MAP infection. As the Ozsvari study shows, milk ELISA-positive cows are those that are less productive and likely to be culled sooner (160.5 days earlier) that their milk ELISA-negative herdmates. In countries with endemic bovine TB, where TB testing of cattle happens regularly, it is important to avoid use of the milk ELISA for at least 60 days after a whole herd TB skin test. TB skin testing seems to increase the rate of false-positive test results (decreases assay specificity).
Milk ELISA testing importantly makes JD control more affordable and thus increases producer participation in national programs like the one in the U.K. However, limitations in diagnostic sensitivity mean that while it is a useful tool for controlling MAP-infections in dairy cattle herds it is not sufficiently sensitive to drive eradication of the infection from herds. For that purpose, more sensitive tests, such as the fecal PCR, are required.
CAN MAP CAUSE CROHN’S DISEASE?
2020-10-23 20:02:56Medical gastroenterologists commonly raise five concerns that seem to argue against a role for MAP in Crohn’s disease.
They are:
- Why is there variability in studies which aim to detect MAP in Crohn’s patients?
- Has MAP fulfilled Koch’s postulates?
- Why does immunosuppressive therapy not worsen Crohn’s Disease if it is caused by MAP, as is seen with MTB (the cause of tuberculosis)?
- Why is the incidence of Crohn’s not higher in at‑risk subgroups, such as veterinarians or farmers?
- If MAP causes Crohn’s, why doesn’t atypical mycobacterial antibiotic therapy (AMAT) cure Crohn’s, and related to that, have RCTs (randomized controlled clinical trials) shown that AMAT is ineffective?
Dr. Gurav Agrawal and colleagues (myself included) published and invited review in the journal Digestive Diseases and Sciences addressing these 5 questions. The article is Open Access (11 pages with 86 references).
Abstract
For decades, Mycobacterium avium subspecies paratuberculosis (MAP) has been linked to the pathogenesis of Crohn’s disease. Despite many investigations and research efforts, there remains no clear unifying explanation of its pathogenicity to humans. Proponents argue Crohn’s disease shares many identical features with a granulomatous infection in ruminants termed Johne’s disease and similarities with ileocecal tuberculosis. Both are caused by species within the Mycobacterium genus. Sceptics assert that since MAP is found in individuals diagnosed with Crohn’s disease as well as in healthy population controls, any association with CD is coincidental. This view is supported by the uncertain response of patients to antimicrobial therapy. This report aims to address the controversial aspects of this proposition with information and knowledge gathered from several disciplines, including microbiology and veterinary medicine. The authors hope that this discussion will stimulate further research aimed at confirming or refuting the contribution of MAP to the pathogenesis of Crohn’s disease and ultimately lead to advanced targeted clinical therapies.
Worth noting:
As evidence that some gastroenterologists think MAP is a cause of Crohn's disease, in the UK, a phase I clinical trial has started to investigate the safety and efficacy of two candidate MAP vaccines in patients with active Crohn's disease (ISRCTN - ISRCTN36126048).
MAP VS DAIRY INDUSTRY – REVIEW
2020-10-15 22:08:42Mary Garvey, Lecturer at the Institute of Technology Sligo, Sligo, Ireland published a review article titled: Mycobacterium Avium Paratuberculosis: A Disease Burden on the Dairy Industry in the journal Animals (11 pages with 58 references).
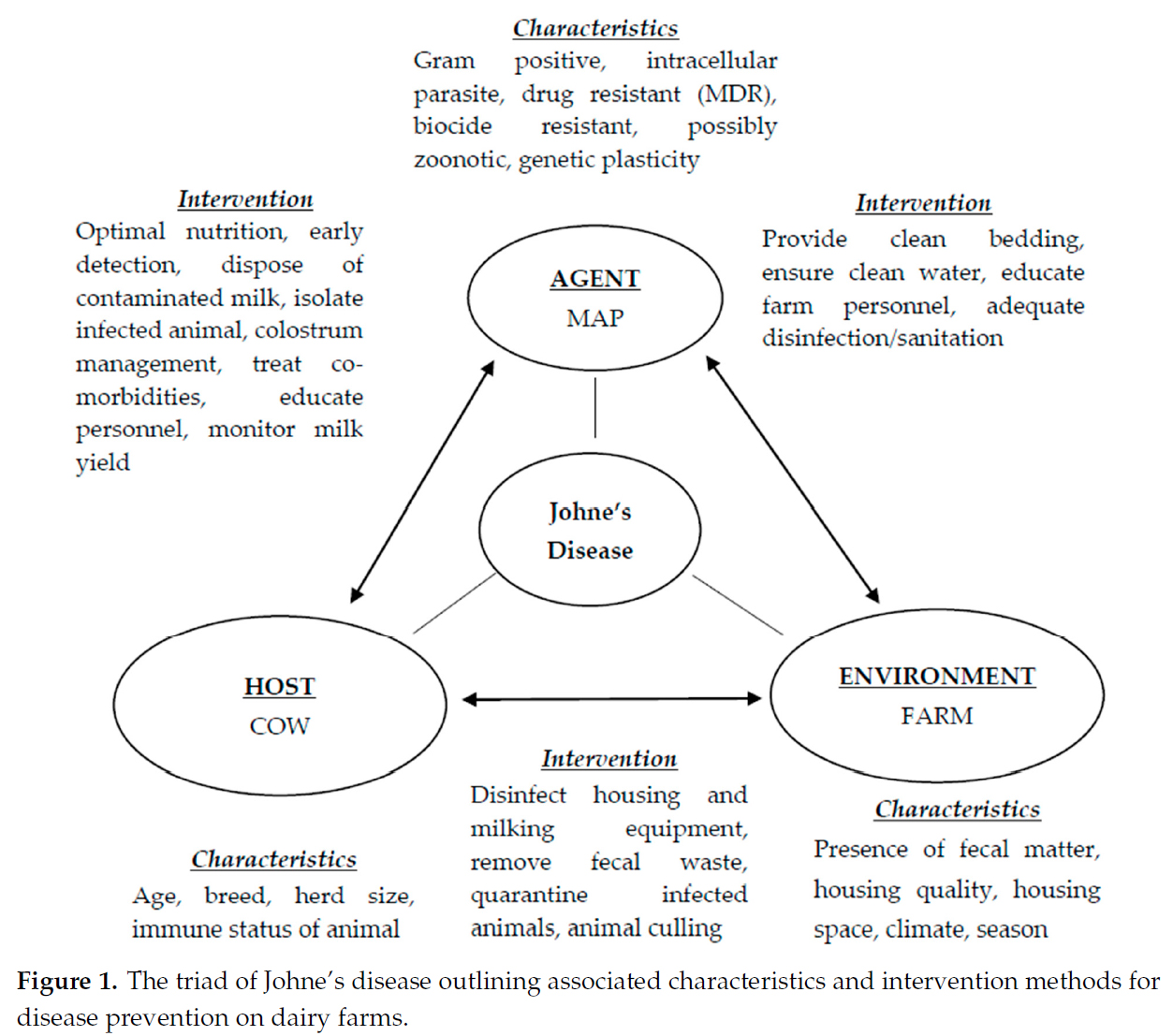
Abstract
Mycobacterium avium paratuberculosis is responsible for paratuberculosis or Johne’s disease in cows, having economic impacts on the dairy industry and a prevalence rate exceeding 50% in dairy herds. The economic burden of Johne’s disease relates to decreased milk production and costs of disease prevention, treatment, and management, while having an economic impact on dairy producers, processors, consumers, and stakeholders of the dairy industry. Determining the true economic impact of the disease is difficult at regional and farm level as symptoms are not evident in subclinically infected animals. At present, the virulence, pathogenicity, persistence, and infectious dose of M. avium paratuberculosis are poorly understood, consequently effective paratuberculosis control measures remain obscure. M. avium paratuberculosis is potentially zoonotic with foodborne transmission a public health risk due to a possible causative link with inflammatory bowel disease in humans. A preventive approach is necessary to reduce the presence of this drug-resistant pathogen in dairy herds and subsequently dairy food. The use of inefficient diagnostic tests coupled with the long latency period of infection results in delayed animal culling and trade of asymptomatic animals, leading to regional transmission and increased disease prevalence. To date, there has been limited success at controlling and treating this terminal endemic disease, leading to significant prevalence rates. This study aims to outline the key factors associated with Johne’s disease while outlining its significant impact on the dairy sector.
Comment: The direct financial costs of Johne’s disease (JD) to dairy producers is small relative to diseases such as mastitis. Hence, without financial incentives most dairy producers conclude that the cost of JD control exceeds the cost of the disease. Therefore, few producers implement JD control programs allowing this chronic, infectious disease to continue spreading within and among dairy herds globally. For more on the epidemiology of JD in dairy cattle read this page of our site.
If, however, MAP is recognized as a zoonotic, food-borne pathogen, causing Crohn’s disease and triggering other so-called autoimmune disease like Type I Diabetes Mellitus, the impact on the dairy industry would be huge.
For more on the MAP as a zoonotic pathogen see this page of our website or for patients, visit the Human Para Foundation website.
Related News
Today, October 15, is the publication anniversary of the landmark paper defining Crohn’s disease (1932). As I do annually, I acknowledge this event in Johnes.org news.
 Eighty-eight years ago, Burrill B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer published a paper titled Regional Ileitis – A Pathologic and Clinical Entity in the Journal of the American Medical Association (vol. 99, no. 16, pp 1323-1329, October 15, 1932). Honoring the importance of this report, the article was later reprinted as a Landmark Article in The Mount Sinai Journal of Medicine (vol 67, no. 3, pp 263-268, May 2006). We provide the original JAMA article here for users interested in reading this influential publication in its original form. Note: the reprinted version in the Mount Sinai Journal of Medicine has better print quality.
Eighty-eight years ago, Burrill B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer published a paper titled Regional Ileitis – A Pathologic and Clinical Entity in the Journal of the American Medical Association (vol. 99, no. 16, pp 1323-1329, October 15, 1932). Honoring the importance of this report, the article was later reprinted as a Landmark Article in The Mount Sinai Journal of Medicine (vol 67, no. 3, pp 263-268, May 2006). We provide the original JAMA article here for users interested in reading this influential publication in its original form. Note: the reprinted version in the Mount Sinai Journal of Medicine has better print quality.
Comment: I appreciate history as I grow older. Also, it is important to read original published reports to avoid misquoting or perpetuating misunderstandings. Interesting note: As described in Wikipedia, Crohn always preferred the medically descriptive terms "regional ileitis" and "regional enteritis" to "Crohn's disease", but he was not able to prevent the appropriation of his name for the disease. It’s worth noting that Johne's disease is also a regional ileitis affecting ruminants.
Without providing much detail, B.B. Crohn’s article mentions efforts to determine if Mycobacterium tuberculosis was involved in the regional ileitis cases he described including culture for M. tuberculosis, inoculation of lymph node homogenates from five patients into guinea pigs, rabbits, and chickens, and acid-fast staining of tissue sections. He concludes that M. tuberculosis was not a cause of these cases of regional ileitis. However, he never mentions the 1913 report by Dalziel or makes any mention of Mycobacterium paratuberculosis or the similarities of regional ileitis in humans to that of cattle, as described by H.A. Johne in 1895. Clearly, Dr. Crohn recognized how the pathology in his afflicted patients resembled that caused by a mycobacterial infection. How might history be different had Dr. Crohn considered the possibility M. a. paratuberculosis (MAP) was the cause?
The picture of B.B. Crohn is credited to Wikipedia.
PARATUBERCULOSIS BOOK – SECOND EDITION
2020-10-09 15:20:04 The second edition of the book titled Paratuberculosis: Organism, Disease, Control has just been published. The editors, Behr, Stevenson and Kapur, have produced a timely follow up to the first book on Paratuberculosis (2010), this new edition is still the only comprehensive text providing both historical context and the latest developments in the field. Examining the epidemiology of paratuberculosis, the organism that causes the disease, and practical aspects of its diagnosis and control, it also addresses the link between paratuberculosis in the food chain and human health implications, including Crohn's disease. The 438 page book contains 23 chapters written by the leading world experts in paratuberculosis (Johne's disease). It is available from Amazon for e-readers for US$147.25 or from Google for US$124.00. The hard cover version has not yet been released for sale but can be pre-ordered from Amazon for US$155.00
The second edition of the book titled Paratuberculosis: Organism, Disease, Control has just been published. The editors, Behr, Stevenson and Kapur, have produced a timely follow up to the first book on Paratuberculosis (2010), this new edition is still the only comprehensive text providing both historical context and the latest developments in the field. Examining the epidemiology of paratuberculosis, the organism that causes the disease, and practical aspects of its diagnosis and control, it also addresses the link between paratuberculosis in the food chain and human health implications, including Crohn's disease. The 438 page book contains 23 chapters written by the leading world experts in paratuberculosis (Johne's disease). It is available from Amazon for e-readers for US$147.25 or from Google for US$124.00. The hard cover version has not yet been released for sale but can be pre-ordered from Amazon for US$155.00
GAME-CHANGING ASSAY FOR VIABLE MAP IN MILK
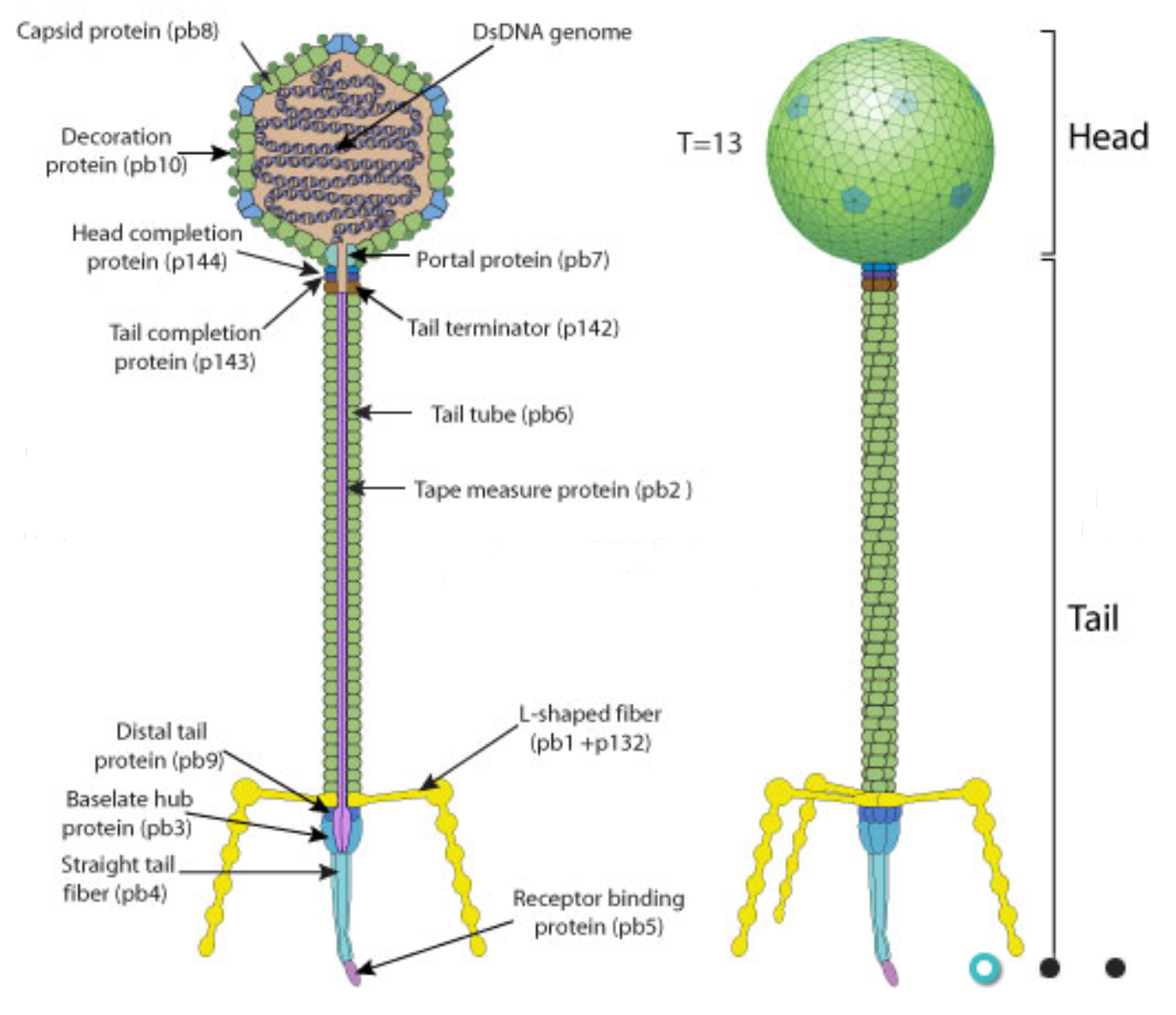
2020-10-02 08:00:55Antonio Foddai and Irene Grant from the Institute for Global Food Security, School of Biological Sciences, Queen’s University Belfast, Belfast, Northern Ireland have created a novel assay to quantify viable MAP in milk (patent pending). Their report, titled “A novel one-day phage-based test for rapid detection and enumeration of viable Mycobacterium avium subsp. paratuberculosis in cows’ milk” appeared September 24 in the journal Applied Microbiology and Biotechnology [Open Access].
In this assay phages (viruses that infect bacteria) are used to both concentrate MAP and to then infect and lyse MAP, allowing for final detection and quantification by qPCR. The total assay time is roughly 7 hours, faster than other phage-based assay systems. The key to the assay is the binding orientation of the phage. The head is bound to the paramagnetic bead allowing the tail to bind to MAP. After binding, the phage then lyses the MAP organism making its DNA available for detection by qPCR.
Diagram of a similar phage from ViralZone.
Abstract
Bacteriophage-based methods for the rapid detection of viable Mycobacterium avium subsp. paratuberculosis (MAP) in veterinary specimens are a recent addition to the Johne’s disease diagnostic toolbox. Here, we report the use of D29 mycobacteriophage-coated tosylactivated paramagnetic beads to capture and concentrate MAP cells from samples (termed phagomagnetic separation, PhMS) and then naturally lyse viable MAP cells (from the inside out) to provide DNA for IS900 qPCR purposes. Transmission electron microscopy confirmed that D29 phages had bound to beads in the correct orientation and that the phage-coated beads captured MAP cells from a suspension. During test optimization, conventional IS900 PCR results were used to subjectively assess the effect of different phage:bead coating ratios, differing amounts of coated beads during PhMS, optimal incubation time post-PhMS to obtain maximal MAP DNA, and the potential benefit of a brief heat shock (55 °C/1 min) prior to IS900 TaqMan qPCR. The limit of detection 50% (LOD50%) of the optimised PhMS-qPCR assay was 10.00MAP cells/50 ml milk (95% CI 1.20–82.83). Finally, in order to demonstrate the new assay’s ability to detect viable MAP in naturally contaminated milk, bulk tank milk samples from 100 dairy farms were tested. Forty-nine (49%) of these tested PhMS-qPCRpositive, with viable MAP numbers detected ranging from 3–126 MAP/50 ml. The novel PhMS-qPCR assay is a sensitive, specific and easy-to-apply phage-based assay for viable MAP, with potential application for milk surveillance or diagnosis of Johne’s disease.
Comment: No other assay system can claim the ability to quantify such low numbers of viable MAP in milk in such a short time. I predict that this assay will reveal that there is far more MAP in raw milk than previously recognized. Hopefully this novel technology also can be applied to other types of clinical samples, e.g. blood samples from Crohn’s disease patients. For more about D29 phages see this Wikipedia page.
« Previous 1 … 5 6 7 8 9 … 18 Next »