MAP & PUBLIC HEALTH
2021-07-15 11:00:47Lauren and John Todd Kuenstner have published an article titled: Mycobacterium avium ssp. paratuberculosis in the Food Supply: A Public Health Issue which appears in the most current issue of Frontiers in Public Health.
Article Description
This article examines the policy implications of Mycobacterium avium subspecies paratuberculosis (MAP) as a zoonotic pathogen and the public health risks posed by the presence of MAP in food, particularly milk products. Viable MAP has been cultured from commercially pasteurized milk in the US. Dairy pasteurization standards and regulations are examined in light of this finding. On the basis of the precautionary principle, the authors suggest options to reduce exposure to MAP, including (1) increased federal authority to regulate pasteurization of all dairy products, (2) modification of pasteurization standards in order to more effectively kill MAP, (3) removal of the Pasteurized Milk Ordinance (PMO) provision that allows states to override federal policy in intrastate dairy sales, and (4) creation of a mandatory Johne's Disease Control Program. These measures would reduce human exposure to MAP and may reduce the risk of diseases associated with MAP.
Comment
The Precautionary Principle is used in EU countries but less so in the US. Wikipedia does an excellent job describing this principle that guides development of many governmental regulations. It is worth reading the entire article, including criticisms are the very end.
JD IN MEXICAN SHEEP
2021-06-23 01:00:12Researchers in Mexico surveyed sheep flocks in the state of Aguascalientes in Mexico. Their findings were published in Abanico Veterinario.
ABSTRACT
With the objective of identifying the presence of Paratuberculosis (PTB), an infectious disease caused by Mycobacterium avium ssp paratuberculosis (MAP), in sheep, through pathological studies, bacterial culture and IS900 PCR, as well as estimating seroprevalence to MAP. The present cross-sectional study, was conducted in 16 different flocks, with the serum of 2415 adult sheep, and analyzed by Enzyme-Linked ImmunoSorbent Assay (ELISA); nine sheep were used with clinical signs suggestive of PTB, from which samples were obtained for the identification studies; obtaining 51.3% of animals seropositive to MAP (1239/2415), in 100% of the herds (16/16); Bacterial isolation and its identification by PCR IS900 were founded in five of the nine cases (5/9) corresponding to 31.25% of the herds (5/16). Confirming the presence of Mycobacterium avium ssp paratuberculosis, and a high frequency of seropositive animals to MAP in flocks of Aguascalientes.

RESUMEN
Con el objetivo de identificar la presencia de Paratuberculosis (PTB), enfermedad infecciosa causada por el Mycobacterium avium subsp paratuberculosis (MAP), en ovinos, a través de estudios anatomopatológicos, cultivo bacteriano y PCR IS900, así como estimar la seroprevalencia a MAP. El presente estudio, de tipo transversal, se realizó en 16 diferentes rebaños con el suero de 2415 animales adultos y analizados por Ensayo por Inmunoabsorción Ligado a Enzimas (ELISA); se emplearon nueve ovinos con signos clínicos sugerentes a PTB, de los cuales se obtuvieron muestras para la realización de los estudios de identificación; obteniendo un 51.3 % de animales seropositivos a MAP (1239/2415), en el 100% de los rebaños (16/16); el aislamiento bacteriano y su identificación por PCR IS900 en cinco de los nueve casos hallados (5/9) correspondiendo al 31.25% de los rebaños (5/16). Conformando la presencia del Mycobacterium avium subsp paratuberculosis, así como una elevada frecuencia de animales seropositivos a MAP en rebaños de Aguascalientes.
COMMENT
Johne’s disease is far more common than most sheep owners realize. Biosecurity regarding animal purchasing is critical to prevention of this economically important disease. This is especially true for sheep breeders. MAP infection prevention is far less costly than MAP infection control. In other words: PREVENTION PAYS. Sheep breeders should only buy animals from flocks that are 100% negative by fecal PCR. Breeders that don’t follow this practice will eventually have sheep that look like the one shown above from publication and then spend years and a lot of money trying to eradicate the infection.
JD VACCINATION OF SHEEP
2021-06-17 16:17:57Australian researchers have published the results of a large scale 10-year effort to control Johne’s disease in sheep, also known as ovine JD (OJD). The study was published in PLOS ONE (38 pages with 106 references). It is one of the most comprehensive and extensive field studies on JD vaccination and the publication is comprehensive in its discussion of OJD and the role of vaccination; highly recommended reading.
ABSTRACT
Background
Mycobacterium avium subsp. paratuberculosis (MAP) causes Johne’s disease (or paratuberculosis), a chronic wasting disease of ruminants and other animals resulting from granulomatous enteritis. There are increasing concerns that MAP is zoonotic. The prevalence of Johne’s disease is increasing worldwide. In an attempt to control an epidemic of ovine Johne’s disease (OJD) in New South Wales (NSW), a government/industry sponsored voluntary vaccination/on-farm management program commenced in 2000. We report herein an observational study of changes in disease prevalence as vaccination progressed, based on abattoir surveillance data for OJD from 1999 to 2009. We also discuss the epidemiological, policy, regulatory, research, economic and sociological elements that contributed to the development of a mature control program, whose aim was to halt the epidemic spread of OJD in a naïve sheep population.
Methods
NSW was divided into areas of “High” (HPA), “Medium” (MPA) and “Low” (LPA) OJD prevalence. A killed whole cell vaccine (Gudair®) was administered to sheep from 2000 to 2009. Trained examiners evaluated the viscera of adult sheep carcasses at slaughter for gross evidence of OJD. MAP infection was confirmed by histopathology.
Principal findings
From 2000–2009, 12 million vaccine doses were administered in NSW (91%; 10.9 million in the HPA). Many of the vaccinated flocks were suffering > 5% annual mortality in adult sheep, with some individual flocks with 10–15% losses attributable to OJD. A total of 7.6 million carcasses were examined (38%; 2.9 million from the HPA). Overall, 16% of slaughter consignments (sheep consigned to the abattoir from a single vendor) were positive for OJD, of which 94% were from the HPA. In the HPA, the percentage of animals with lesions attributable to OJD at slaughter fell progressively from 2.4% (10,406/432,860) at commencement of vaccination in 2000 to 0.8% (1,573/189,564) by 2009. Herd immunity from vaccination in the HPA was estimated at 70% by 2009, the target commonly espoused for an effective control program based on vaccination. This coincided with a progressive decrease in reports of clinical disease and mortalities in vaccinated flocks.
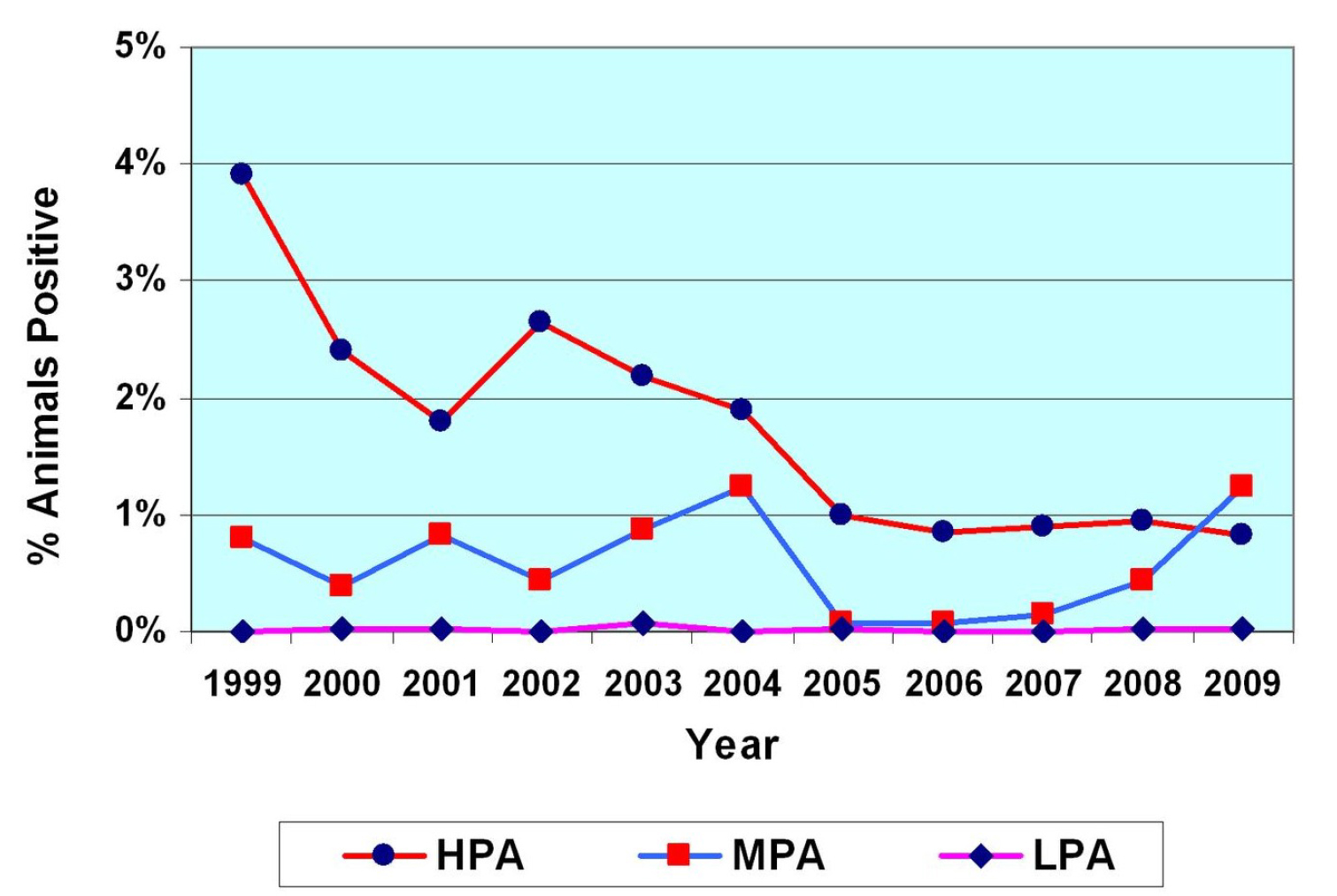
Significance
We show a decrease in the prevalence of lesions attributable to OJD in NSW concomitant with initiation of voluntary vaccination, on-farm management plans, abattoir monitoring and feedback of animal prevalence data to sheep producers. We conclude that a target of ≤ 1% regional prevalence of OJD affected sheep at slaughter is achievable using these interventions.
COMMENTS
As the authors noted, the decline in OJD animal-level prevalence was achieved “by the combination of voluntary vaccination, abattoir monitoring, feedback of surveillance data to sheep producers and the implementation of appropriate disease management strategies on farm”. The study was not designed to define the extent to which each of these practices contributed to OJD control, although there is a tendency to give the vaccine most of the credit.
Only 7 countries (31.8% of 22 countries with JD control programs) use vaccination as part of their JD control program (Whittington, 2019). This is due in part to conflicting data on the efficacy of vaccination, interference with TB testing and eradication programs, and the hazards of using the current vaccines. Presently, the widest use of JD vaccines is in countries with a significant small ruminant animal agriculture and then primarily in commercial production systems, as opposed to breeder flocks. This is largely due to economics, i.e., other JD control methods are deemed not affordable.
This important publication shows the economic value of vaccination as part of a comprehensive JD control effort at a regional or national level. Having lost the opportunity in most countries to eradicate JD before it became endemic, vaccination may become necessary for protection of both animal and human health. However, there is significant room for improvement in the safety and efficacy of current JD vaccines.
MAP IN DOMESTIC WATER
2021-06-01 16:02:56 Portuguese researchers found high levels of MAP in domestic water supplies in parts of Portugal with a high incidence of inflammatory bowel diseases, such as Crohn’s disease, in humans. The article by Sousa and colleagues was published in AIMS Microbiology.
Portuguese researchers found high levels of MAP in domestic water supplies in parts of Portugal with a high incidence of inflammatory bowel diseases, such as Crohn’s disease, in humans. The article by Sousa and colleagues was published in AIMS Microbiology.
ABSTRACT
Mycobacterium avium subsp. paratuberculosis (MAP) may play a role in the pathology of human inflammatory bowel disease (IBD). Previously, we found a high frequency (98% in patients with active disease) of MAP DNA detection in the blood of Portuguese Crohn’s Disease patients, suggesting this cohort has high exposure to MAP organisms. Water is an important route for MAP dissemination, in this study we therefore aimed to assess MAP contamination within water sources in Porto area (the residential area of our IBD study cohort).
Water and biofilms were collected in a wide variety of locations within the Porto area, including taps connected to domestic water sources and from municipal water distribution systems. Baseline samples were collected in early autumn plus further domestic water samples in early winter, to assess the effect of winter rainfall. DNA was extracted from all 131 samples and IS900-based nested PCR used to assess the frequency of MAP presence.
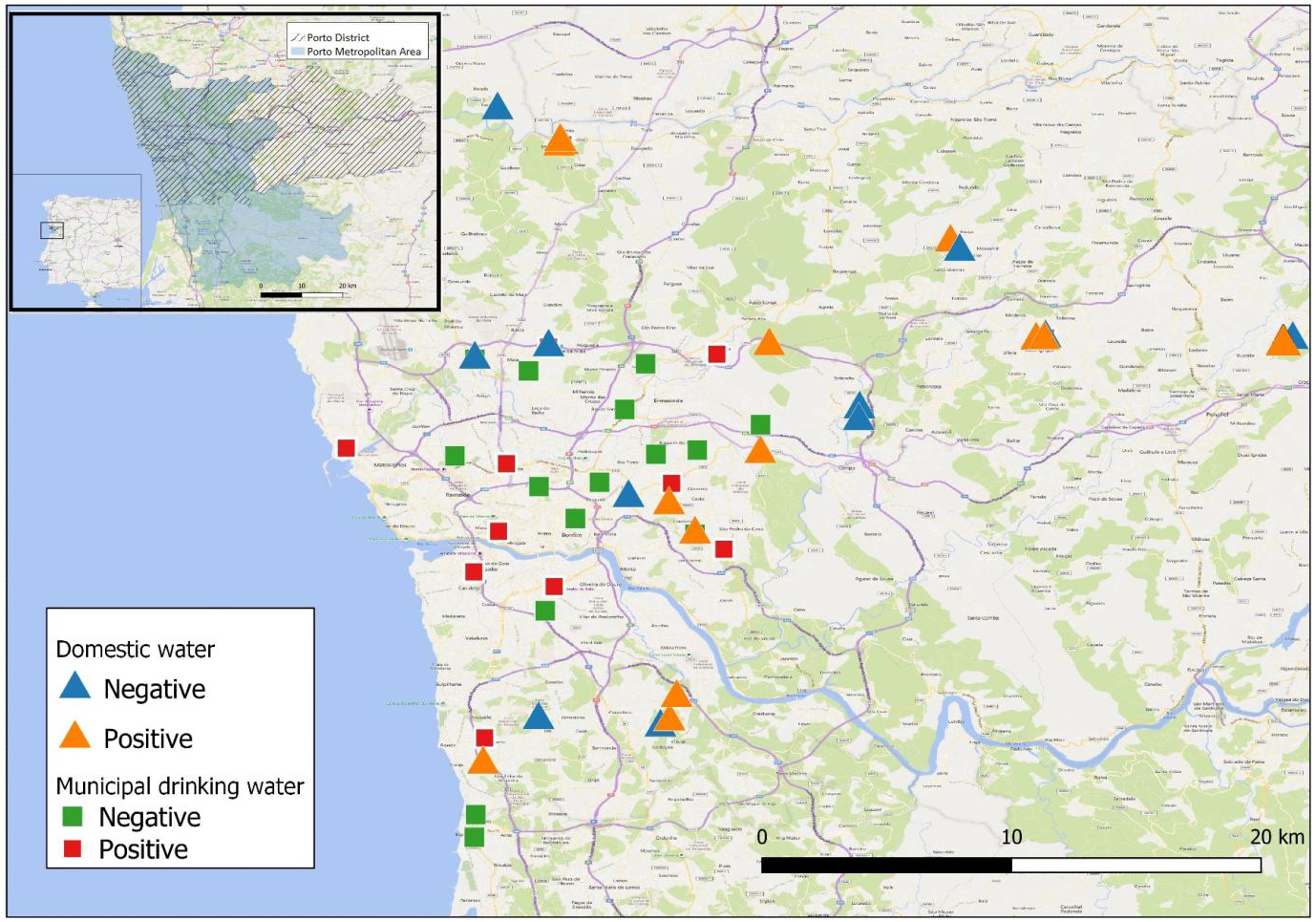
Our results show high MAP positivity in municipal water sources (20.7% of water samples and 41.4% of biofilm samples) and even higher amongst domestic sources (30.8% of water samples and 50% of biofilm samples). MAP positivity in biofilms correlated with positivity in water samples from the same sources. A significantly higher frequency of MAP-positivity was observed during winter rains as compared with samples collected in autumn prior to the winter rainfall period (61.9% versus 30.8%). We conclude that domestic and municipal water sources of Porto region have a high burden of MAP contamination and this prevalence increases with rainfall. We hypothesize that human exposure to MAP from local water supplies is commonplace and represents a major route for MAP transmission and challenge which, if positively linked to disease pathology, may contribute to the observed high prevalence of IBD in Porto district.
COMMENT
MAP is spilling over from animals to humans through contaminated food and water leading to human disease. In other words, MAP is a zoonotic infection, and it is a global problem. The most effective means of protecting human health is to control the infection in food-producing animals. Doing so will not only improve human health, it also will improve animal health and welfare as well as farm profitability. Seems like a “no-brainer”.
PROPHETIC WORDS FROM 1922
2021-05-22 01:00:09 Ninety-nine years ago today, two University of Wisconsin faculty published a seminal article on Johne’s disease. The full article is provided here for Johne’s history enthusiasts. Although published so long ago, the descriptions of the pathology and epidemiology of the disease are remarkably accurate. Today’s Johne’s news highlights some of the prophetic statements made by those authors.
Ninety-nine years ago today, two University of Wisconsin faculty published a seminal article on Johne’s disease. The full article is provided here for Johne’s history enthusiasts. Although published so long ago, the descriptions of the pathology and epidemiology of the disease are remarkably accurate. Today’s Johne’s news highlights some of the prophetic statements made by those authors.
“Johne’s disease is one that is not at all widespread in Wisconsin or in any part of our country at present. It does occur, however, and as the years go by it will become more and more common and will place a greater tax on the cattle industry unless some consideration is given to it by those engaged in the raising and sale of cattle.”
 “The affected animals lose flesh very slowly until they become virtually walking skeletons. The unthrifty condition of animals, in spite of abundant feed, is occasioned in part by this disease.”
“The affected animals lose flesh very slowly until they become virtually walking skeletons. The unthrifty condition of animals, in spite of abundant feed, is occasioned in part by this disease.”
“…it is likely to be present in pure bred herds from which animals are being sold in great numbers. A few such distributing centers will thus spread with a constantly increasing rapidity unless more attention is paid too it that is done at present.”
“The aim of this bulletin is to call the attention of veterinarians and breeders to Johne’s disease, which, it is felt, is not recognized by many, in order that steps may be taken to prevent its introduction into still healthy herds, and to gradually eliminate it from affected herds.”
These statements were published by B.A. Beach and E.G. Hastings in 1922 in the Wisconsin Bulletin, a publication by the Wisconsin Agriculture Experiment Station. Their warnings remain true today and their aim, to raise awareness, is the same aim as that of this website.
Fast forward 99 years and, based on USDA survey data from 2007, we find that over 90% of U.S. dairy herds are now MAP-infected (Lombard et al., 2013), and the situation in most other major dairy-producing countries is similar. Millions of animals have died or been culled from herds due to this disease. Millions of dollars in farm income are lost annually because we failed to heed these warnings. And, we now find the cause of Johne’s disease, MAP, spilling into humans through contaminated food and water.
- More indications that JD is common and MAP is in raw meat:
A Georgia, USA a survey of cattle sold at sale barns using ELISA found that 9.58% of dairy cattle and 3.95% of beef cattle tested ELISA-positive. Given the accuracy of ELISAs, the actual infection rates are roughly double these figures (Pence, 2003). Most of these cattle were headed to slaughterhouses (abattoirs) and then into the food supply. - A survey of culled dairy cows in Atlantic Canada and Maine, USA found that 16.1% of the cows were culture-positive for MAP. The meat from these animals also was headed into the human food chain (McKenna, 2004).
- A Colorado, USA study of 40 cull dairy cows found that 21 had MAP disseminated infection. Moreover, 57% (12/21) of cows with disseminated infection had average to heavy body condition and no diarrhea. Cows with disseminated infection had no to minimal gross pathologic evidence of infection in 37% (8/21) of cases. Only 76% (16/21) of cows with disseminated infection had positive historical ELISA results and only 62% (13/21) had a positive ELISA at slaughter (Antognoli, 2008). Thus, further verifying that ELISAs underestimate the number of MAP-infected cows with disseminated MAP infections that enter the food supply.
Side note:
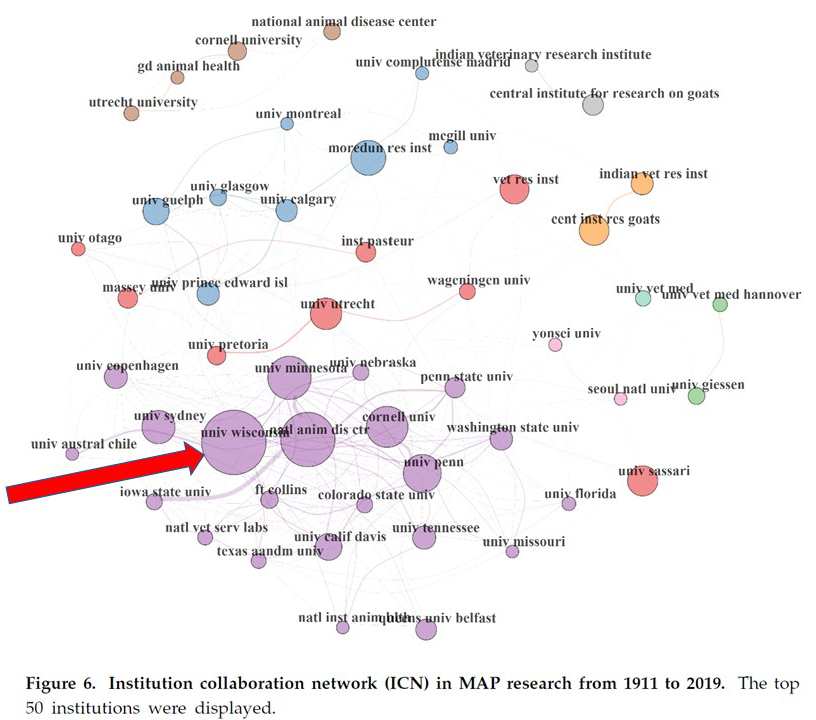
The University of Wisconsin (UW) has remains a leader in Johne’s disease diagnosis and research. Ekundayo and Okoh provided a review of paratuberculosis literature published from 1911 to 2019 (Microoganisms, 2020). The graphic below from their publication indicates, by size of the circle, the number of scientific publications and collaborations between UW and other institutions working on paratuberculosis. The purple circle for the University of Wisconsin is the largest and most interconnected. This website is a legacy of the UW tradition in paratuberculosis research and outreach.
SAMPLE POOLING SAVES MONEY
2021-03-26 16:58:46Surveillance of cattle herds for MAP infections can be costly if individual cows are tested by fecal PCR. Some countries, such as the U.S., have adopted methods allowing pooling of fecal samples from 5 cattle before doing culture of PCR testing. If the pooled fecal sample tests PCR-negative, then all cattle contributing to that pooled sample are considered PCR-negative.
Australian researchers published a simulation modelling study indicating that the pool size can be increased to 10. Their study was published in the journal Preventive Veterinary Medicine.
ABSTRACT
Johne’s disease is a chronic intestinal disease affecting livestock. It leads to the shedding of Mycobacterium avium subspecies paratuberculosis (MAP) in the faeces, wasting and eventually death, with animal welfare, economic, and trade implications. The Johne’s Beef Assurance Scheme, used in Australia to determine the risk of Johne’s disease on beef properties and facilitate trade, is based on testing a subset of the herd with pooled faecal quantitative PCR. This study aimed to model the herd-sensitivity of pooled faecal testing under different Australian farming scenarios. Animals from simulated herds were randomly sampled and allocated into their respective pools. Each tested pool was provided a test outcome, with herd-sensitivity estimated as the probability of detecting a truly infected herd. The models simulated the test performance for the ‘Sample’ and ‘Check’ tests used in the assurance schemes (recommended sample sizes of 300 and 50, respectively) for a range of herd sizes, infection prevalence and MAP faecal shedding levels for the pool sizes of 5, 10, 15 and 20. Sensitivity and specificity input values of each pool size were obtained from a previous laboratory investigation. The herd-sensitivity estimate increased with herd size and infection prevalence levels, regardless of the pool size. Higher herd-sensitivity was also achieved for testing scenarios involving larger sample sizes. A pool size of 10 achieved similar herd-sensitivity to that of the current pool size for the majority of the Sample test and Check test scenarios. This was particularly evident when pool-specificity was assumed to be perfect. The overall herd-sensitivity of the Check test was very low for all infection prevalence levels and pool sizes, but it more than doubled, when the sample size increased from 50 to 100 animals (11% versus 26% for a herd size of 500 cattle with a 2% infection prevalence). The results show that the majority of beef producers participating in the assurance scheme can benefit from using a larger pool size for the pooled faecal quantitative PCR testing of their herd, in comparison to the pool size currently used.
COMMENTS
Kalis et al. (2000) first demonstrated that fecal sample pooling (5/pool) was effective at detecting MAP-infected dairy herds. Importantly, he showed that samples should be pooled based on animal age. He called this “strategic pooling”. The importance of this cannot be stressed enough. Animals born around the same time experience similar risks of acquiring a MAP infection. Therefore age-based (strategic) pooling helps insure that the least number of sample pools test positive and thus the fewest number of individual cattle require follow-up PCR testing.
Wells et al. (2002) compared dairy cattle fecal pools of 5 and 10 and concluded that pools of 5 were slightly more sensitive than pools of 10 when using fecal culture as the diagnostic test. This was a small study that used feces from only 10 cows to construct various fecal combinations in pools.
Van Schaik et al. (2007) collected 50 fecal samples from each of 12 Chilean dairy herds and compared pools of 5 and 10 samples finding that pools of 10 were as good or better than pools of 5 at detecting MAP-infected dairy herds.
It is challenging for veterinary diagnostic laboratories to change protocols but perhaps the time has come to consider using age-based pooling of 10 fecal samples in cattle herds that have a very low or zero rate of MAP-infections. Unless a herd is trying to meet US. or other Johne's disease program standards for herd classification, there is no mandate to test fecal samples in pools of 5. Standards may differ in other countries.
Below are cost calculations for a herd of 100 cattle tested by fecal PCR using pools of 5 or 10 cattle/pool. Only laboratory fees are calculated, and prices are based on the rates posted on the Wisconsin Veterinary Diagnostic Laboratory (WVDL) website 26-MAR-2021 (individual feal PCR @ $30.89 and pooled PCR @ $35.02). It does not include the $10 accession fee, veterinary costs, or sample shipping. Costs are rounded to the nearest U.S. dollar. Note: The WVDL has no extra cost for Johne’s disease tests for samples originating from states or than Wisconsin.
For comparison, the cost of testing 100 cattle individually by fecal PCR would be $3,502.
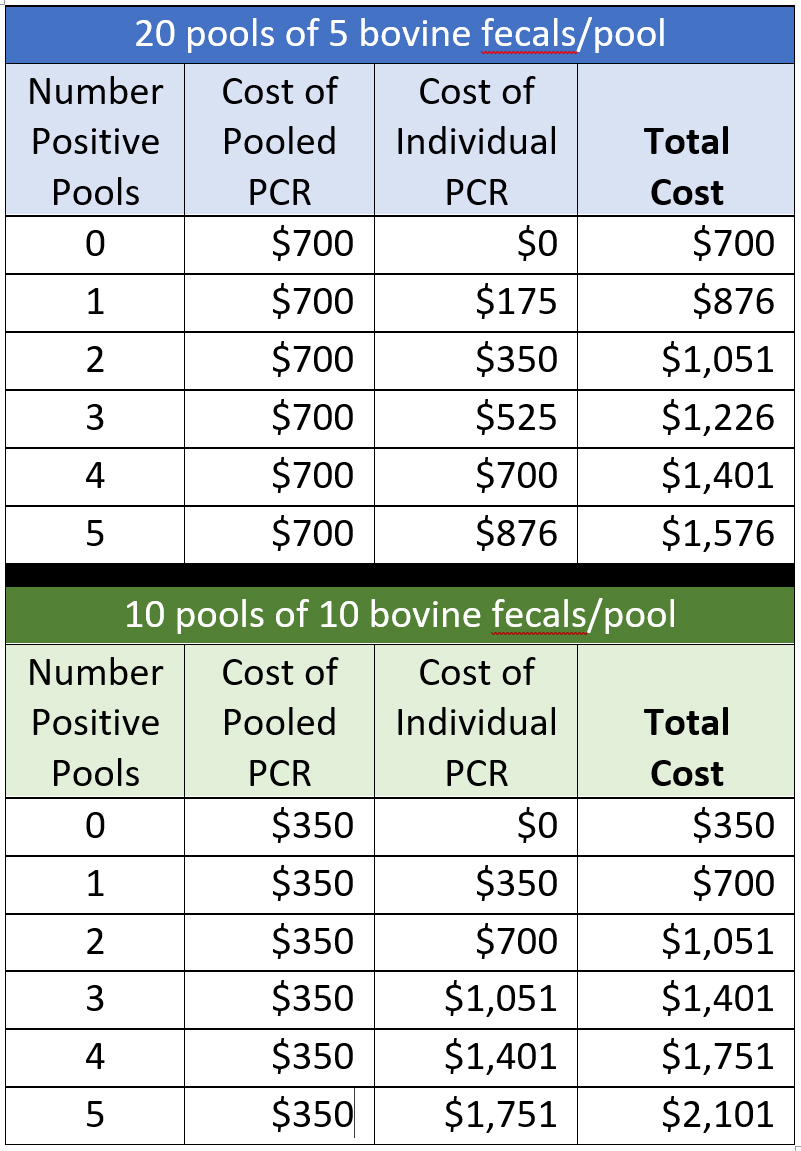
This table illustrates that:
- Sample pooling can save money. Even if 5 fecal pools test PCR-positive the total cost for testing a herd of 100 cows would be less than testing each cow individually by fecal PCR.
- Sample pooling is most advantageous for herds with a low MAP infection prevalence, i.e., few PCR-positive pools.
It is important to stress that to have the fewest number of positive pools, samples should be pooled based on animal age. This will tend to group fecal samples from MAP-infected cows into the same sample pool results in in fewer pools requiring individual fecal sample testing.
How does this compare to testing by ELISA (blood sample testing)?
The cost for testing a herd of 100 cows by ELISA would be $600 at current WVDL prices. In most instances, cows testing ELISA-positive would have to be retested by individual fecal PCR to confirm the diagnosis. This makes the per head cost of doing pooled fecal PCR equal to or less than that of testing by ELISA for low prevalence herds. This is especially true if pools of 10 are tested. Given that the fecal PCR is roughly 2 to 3 times more sensitive than the ELISA, herd owners get a better return on their investment in Johne’s disease diagnostics by using fecal PCR on pooled samples; no need to resample and test ELISA-positive cows by fecal PCR and greater confidence in that the PCR-negative cattle are truly not MAP-infected.
Not all laboratories offer pooled fecal PCR testing and prices vary considerably among veterinary diagnostic laboratories. Talk to your herd veterinarians and consult with testing laboratories before sending samples. This website provides a list of laboratories that are USDA-approved to perform pooled fecal testing: scroll down to the PDF file named “Johne’s Disease-pooling methods”.
What about sheep and goats?
Sample pooling studies have not been reported for goats, but cattle-based studies are a good guide. In Australia, fecal samples from 50 sheep are pooled before PCR testing.
WHY JD CONTROL FAILS
2021-03-19 01:00:02Swiss veterinarians followed Johne’s disease (JD) control programs in 7 beef cattle and 10 dairy herds over a 3-year period. They documented the degree to which owners adopted JD control recommendations and monitored the level of MAP infection in the herds primarily by fecal culture with some supplemental testing by ELISA and fecal PCR. This study was reported in the journal PLOS ONE and is Open Access.

ABSTRACT
Various measures have been advocated for the control of Johne’s disease (caused by Mycobacterium avium subsp. paratuberculosis, MAP) in different countries. Farmers’ compliance has been reported to be variable depending on disease prevalence and incentives to participate in control programs. After the prevalence of MAP shedding and risk factors for within-herd spread of MAP were assessed in 17 Swiss cattle herds (10 dairy and 7 beef), general and herd-specific recommendations were given to the farmers to reduce MAP transmission within the herd. Participation in the study and implementation of control measures were voluntary, no financial incentives were provided for the realization of control measures. After a 3-year period of monitored observation including biannual farm visits and discussion of the situation, the implementation of the recommended control measures and their effect on prevalence of MAP shedding were evaluated. Implementation of recommended general and farm-specific control measures was only partially realized. Neither the number of animals tested positive (before or during the study) nor the farmers’ knowledge about paratuberculosis were significantly associated with their compliance for the implementation of management changes. The apparent within-herd prevalence remained constant despite limited implementation of control measures, and no particular group of control measures was found to be associated with changes in prevalence. Farmers’ compliance for the implementation of control measures to reduce the impact of Johne’s disease in infected farms was very limited under Swiss farming conditions in the frame of voluntary participation in a research project. These results indicate that the losses associated with paratuberculosis in Swiss dairy and beef operations are not estimated to be high enough by the farmers to justify important efforts for control measures, and that incentives may be necessary to achieve efficient implementation of such measures.
CONCLUSIONS (from the publication)
The managers of infected Swiss dairy and beef herds were reluctant to implement control measures to minimize MAP spread in the frame of their voluntary participation in a research project conducted over a period of three years. Except for culling test-positive animals, implementation rates were low for most proposed measures, and some of those that were implemented at the beginning of the period of observation were discontinued in the course of the study. The farmers mostly implemented cheap and easy measures, while changes impacting farm structure or their routines more deeply, e.g. improving hygiene in the calving area or minimizing animal purchase, were rarely realized. The farmers did not observe relevant economic losses due to PTB in their herds and therefore did not expect an immediate reward for expenditures in time, efforts and money. These observations suggest that incentives for a consequent implementation of control measures should be included in future programs to increase the compliance of the managers of herds infected with PTB and thus improve the chances of successfully controlling the disease.
COMMENT
Despite many studies documenting the cost of Johne’s disease to both dairy and beef cattle operations, these costs are not readily apparent to producers. And, if these producers are expected to shoulder 100% of the cost of controlling Johne’s disease but perceive little financial benefit, programs will not likely succeed, as the Swiss study shows. When programs have broad industry support, and the meat and milk processors who buy raw products from producers pay a premium for products from herds that are successfully controlling Johne’s disease, control program costs are shared equitably, and programs are more likely to succeed. This is the basis of the most recent national programs underway in the UK and the Republic of Ireland. JD control program costs may be passed along to consumers in the form of slightly higher retail product prices, but consumers will benefit because JD control programs result in less MAP entering the food chain making the finished products safer to consume.
As a reminder:
- MAP is found in raw milk and meat.
- MAP survives pasteurization and is found in retail milk.
- MAP survives cooking unless the meat is cooked until it is “well-done”.
- MAP is routinely found in people with Crohn’s disease (a human disease resembling JD).
- People with Crohn’s disease clinically improve when treated with anti-MAP antibiotics; some have been fully cured.
MAP is likely a food-borne zoonotic pathogen affecting people worldwide. Just as for other zoonotic pathogens found in domestic animals, such as tuberculosis and brucellosis, control programs that benefit both animal and human health begin on the farm. This is fundamental to the One Health philosophy.
- Read more about MAP in food here.
- Read more about the zoonotic potential of MAP here or watch the video on this page.
JD IN BEEF CATTLE LECTURE
2021-03-12 13:10:55
Beef cattle producers and their herd veterinarians commonly ask:
- What is the best test to help control JD in beef cattle?
- What should I do with cattle that test ELISA-positive
- How can I avoid buying MAP-infected cattle?
- Which test should I use to certify my herd is free of Johne’s disease?
- What are my first steps in controlling Johne’s disease?
- Should I use the ELISA for JD on blood samples of PCR on fecal samples?
- Will JD control improve my farm profitability - what's the evidence?
- How common are national JD control programs and how to they work?
These and many more questions are addressed in a new 60-minute lecture titled “Johne’s Disease in Beef Cattle” now available on the Johnes.org website. This lecture is based on a a recent continuing education program for veterinarians. You will also find on this same web page lectures titled Johne’s disease in dairy cattle, parts-1 and 2” “Johne’s Disease in Goats” and “MAP is a Zoonotic Pathogen”.
NEW ASSAY FINDS MORE MAP IN MILK
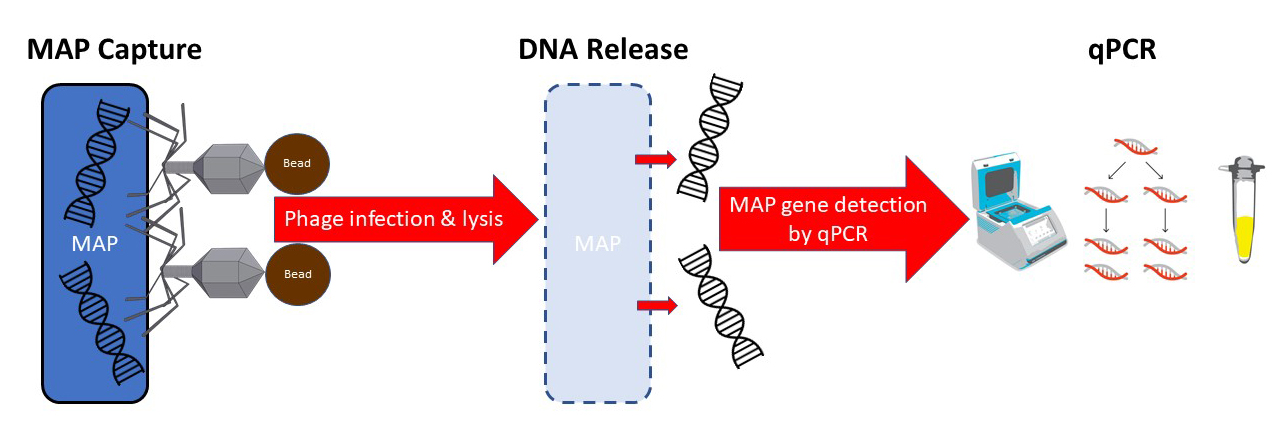
2021-03-05 01:00:01Antonio Foddai and colleagues have developed a novel assay for detection of viable MAP in milk. The assay uses bacteriophages (viruses that attack bacterial cells) attached to magnetic beads to capture MAP in milk samples and then detect the presence of MAP by PCR. While these phages bind to both living and MAP cells, they only then infect and lyse live MAP. Thus, this assay only detects live MAP, just as do conventional culture methods. This new assay has higher diagnostic sensitivity and specificity than the most commonly used assay on milk today, the milk ELISA.
The article, published in the Journal of Dairy Science, is not Open Access, but is available for the next 40 days.
ABSTRACT
Bulk tank milk samples from 392 Northern Ireland dairy farms and individual milk from animals (n = 293) on 4 of these farms were tested by a novel phagomagnetic separation (PhMS)-quantitative (q)PCR assay able to detect and quantify viable Mycobacterium avium ssp. paratuberculosis (MAP), to demonstrate its potential utility as a milk surveillance tool. Viable MAP were detected in 26.5% of the bulk tank milks, with MAP contamination levels ranging from 1 to 8,432 MAP/50 mL of milk; less than 2% of farms had MAP contamination levels >100 MAP/50 mL in their bulk tank milk. Follow-up PhMS-qPCR testing of milk from individual animals on 4 farms that had the highest numbers of MAP in their bulk tank milks indicated that 17 to 24% of animals in each herd were shedding viable MAP in their milk. Mean MAP numbers detected ranged between 6.7 and 42.1 MAP/50 mL of milk. No significant correlation was observed between the detection of viable MAP in bulk or individual milks by PhMS-qPCR and parallel milk ELISA results, or between PhMS-qPCR results and any other milk recording results (somatic cell count, total bacterial count, % butterfat, or % protein). Viable MAP was detected by IS900 qPCR in 52 (85.2%) Pozzato broth cultures of 61 PhMS-qPCR-positive individual milks after 12 wk of incubation, suggesting few PhMS-qPCR results were false positives. The mean sensitivities of the PhMS-qPCR assay and milk ELISA applied to individual milks were estimated by Bayesian latent class analysis to be 0.7096 and 0.2665, respectively, and mean specificities were similar (0.9626 and 0.9509). Our findings clearly demonstrate that the novel PhMS-qPCR assay could be a useful milk surveillance tool for dairy processors, or a milk monitoring tool for Johne's disease control or milk quality assurance programs.
COMMENT
Phage-based assays for MAP have been described previously. This is the first time, however, that the phage has been linked to a magnetic bead allowing retrieval and concentration of MAP using magnets. In addition to binding to MAP, the phage then infects and lyses the live MAP bacterial cell, releasing its DNA allowing detection by qPCR technology. My schematic of the process appears below.
The head of the phage is linked to a magnetic bead. The tail of the phage binds to live mycobacteria. A magnet then pulls all of the beads+phage+MAP out of the sample.
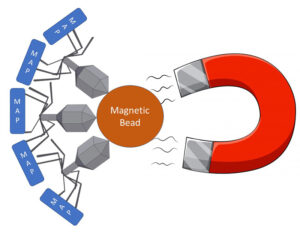
After collecting the beads on a magnet, the phage then infects and lysis the live mycobacterial cells releasing their DNA. A qPCR specific for MAP then detects the pathogen. The phage-bead-magnet combination makes the assay very sensitive and the qPCR part of the assay makes it very MAP-specific.
Phage amplification assays for detection of mycobacterial infections were recently reviewed in the journal Microorganisms. This excellent 28 page Open Access article with 97 references provides a deeper understanding of phage-based mycobacterial detection technology but does not address this new twist: the use of phages to both capture and lyse the mycobacteria.
Actiphage is a commercial test, based on phage technology, for diagnosis of bovine tuberculosis and Johne's disease. Dr. Cath Rees (University of Nottingham) will be presenting a talk “Use of Actiphage to improve our understanding of mycobacterial diseases” on the 23rd March at 2pm as part of a virtual virology conference. Registration for the event is free.
The Wisconsin Connection
Fun factoid: Dr. Tom Brock, University of Wisconsin-Madison Professor, discovered a bacterium he named Thermus aquaticus, which grows at temperatures over 70 degrees C (158 degrees Fahrenheit) . Parts of its metabolic machinery, including the enzyme Taq polymerase, needed to assemble strands of DNA, tolerates boiling. In 1989, Science named Taq polymerase its first “molecule of the year,” because it enabled polymerase chain reaction (PCR), an essential tool for molecular biologists. Today's #1 test for COVID also depends on PCR.
The world owes a big "thank you" to Dr. Tom Brock.
DETECTION OF MAP-INFECTED DAIRY HERDS
2021-02-25 01:00:58 Vera Fichtelova and colleagues from the Veterinary Research Institute, Brno, Czech Republic published a study on the optimal method for detecting MAP-infected dairy herds using environmental fecal sampling. They found that fecal samples collected from the floor of the milking alleys (paths for cows to come into the milking area close to the entrance into the milking parlor holding pens) tested by qPCR (also known as real-time PCR) was a simple and effective method for detection of dairy herds with a significant MAP infection rate.
Vera Fichtelova and colleagues from the Veterinary Research Institute, Brno, Czech Republic published a study on the optimal method for detecting MAP-infected dairy herds using environmental fecal sampling. They found that fecal samples collected from the floor of the milking alleys (paths for cows to come into the milking area close to the entrance into the milking parlor holding pens) tested by qPCR (also known as real-time PCR) was a simple and effective method for detection of dairy herds with a significant MAP infection rate.
ABSTRACT
The objective of the present study was to evaluate the suitability of environmental sampling to screen Czech dairy herds to detect Mycobacterium avium ssp. paratuberculosis (MAP) and to find the most convenient location for the MAP detection in the lactating cow area. Environmental samples (ES, n = 72) from milking parlour holding pens (n = 19), milking alleyways (n = 19) and free-stall alleyways (n = 34) from 19 herds were simultaneously tested to detect MAP by a quantitative PCR (qPCR) and bacterial culture. Eight and thirteen samples from the milking parlour holding pens, twelve and eleven samples from the milking alleyways and eleven and eighteen samples from the free-stall alleyways were qPCR and culture positive, respectively. A 4.6 times higher probability of being culture positive than qPCR positive was detected for the assessable MAP detection results from the free-stall alleyways [P = 0.008 6, odds ratio (OR) = 4.572 8)] and no association was found between the results from the milking parlour holding pens (P = 0.191 4) and the milking alleyways (P > 0.999 9) and the diagnostic method used. The percentage of qPCR-positive samples in the tested locations was detected for the milking alleyways (63.2%), free-stall alleyways and milking parlour holding pens. The herd infectious status was in agreement with 16 (84.2%), 14 (73.7%) and 12 (63.2%) qPCR results from the milking alleyways, free-stall alleyways (32.4%) and milking parlour holding pens (42.1%), respectively. No statistically significant differences were detected for these results (P = 0.396 1). MAP was detected by the qPCR and bacterial culture in all three locations where the ES were collected. We suggest an environmental sampling followed by MAP detection by qPCR as an easy-to-perform time-saving protocol for MAP screening in Czech dairy herds. Although the milking alleyways seem to be the most convenient location for the environmental sampling, this assumption was not statistically supported.
COMMENT
The first step toward Johne’s disease control in commercial dairy herds (herds in the business of selling milk, not breeding animals) is to establish which herds have a significant MAP infection rate. Environmental fecal samples tested by qPCR provides a rapid, low-cost methods for testing the whole dairy herd.
The environmental fecal sample methods described in the U.S. Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program are much more difficult to perform. Page 33 of that document states: “From each dairy farm, collect two composite environmental fecal samples tested by an MAPDT (MAP detection test meaning culture of PCR) from each of the following locations on the farm: manure concentration areas (cow housing alleyways or gutters), manure storage areas (lagoons, piles, pits, or manure spreader), and another manure concentration area (sick cow pens or other cow alleyways and travel-ways). A total of six samples should be collected for submission to the diagnostic laboratory.”
The Czech system may allow more herds to be tested at less cost and therefore may be preferable to the U.S. system. However, the critical question is whether the herd-level sensitivity is comparable between the U.S. method and the newly described Czech system and also the comparative utility of the two methods. A testing system that is too onerous and therefore not used is not useful.
« Previous 1 … 3 4 5 6 7 … 18 Next »